当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A combined crossed molecular beams and computational study on the formation of distinct resonantly stabilized C5H3 radicals via chemically activated C5H4 and C6H6 intermediates†
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-03-08 00:00:00 , DOI: 10.1039/c8cp00357b Aaron M. Thomas 1, 2, 3, 4 , Michael Lucas 1, 2, 3, 4 , Long Zhao 1, 2, 3, 4 , Jerid Liddiard 1, 2, 3, 4 , Ralf I. Kaiser 1, 2, 3, 4 , Alexander M. Mebel 4, 5, 6, 7
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-03-08 00:00:00 , DOI: 10.1039/c8cp00357b Aaron M. Thomas 1, 2, 3, 4 , Michael Lucas 1, 2, 3, 4 , Long Zhao 1, 2, 3, 4 , Jerid Liddiard 1, 2, 3, 4 , Ralf I. Kaiser 1, 2, 3, 4 , Alexander M. Mebel 4, 5, 6, 7
Affiliation
|
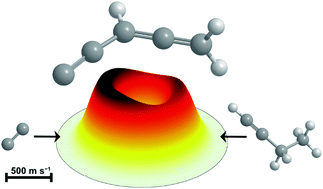
The crossed molecular beams technique was utilized to explore the formation of three isomers of resonantly stabilized (C5H3) radicals along with their d2-substituted counterparts via the bimolecular reactions of singlet/triplet dicarbon [C2(X1Σ+g/a3Πu)] with methylacetylene [CH3CCH(X1A1)], d3-methylacetylene [CD3CCH(X1A1)], and 1-butyne [C2H5CCH(X1A′)] at collision energies up to 26 kJ mol−1via chemically activated singlet/triplet C5H4/C5D3H and C6H6 intermediates. These studies exploit a newly developed supersonic dicarbon [C2(X1Σ+g/a3Πu)] beam generated via photolysis of tetrachloroethylene [C2Cl4(X1Ag)] by excluding interference from carbon atoms, which represent the dominating (interfering) species in ablation-based dicarbon sources. We evaluated the performance of the dicarbon [C2(X1Σ+g/a3Πu)] beam in reactions with methylacetylene [CH3CCH(X1A1)] and d3-methylacetylene [CD3CCH(X1A1)]; the investigations demonstrate that the reaction dynamics match previous studies in our laboratory utilizing ablation-based dicarbon sources involving the synthesis of 1,4-pentadiynyl-3 [HCCCHCCH(X2B1)] and 2,4-pentadiynyl-1 [H2CCCCCH(X2B1)] radicals via hydrogen (deuterium) atom elimination. Considering the C2(X1Σ+g/a3Πu)–1-butyne [C2H5CCH(X1A′)] reaction, the hitherto elusive methyl-loss pathway was detected. This channel forms the previously unknown resonantly stabilized penta-1-yn-3,4-dienyl-1 [H2CCCHCC(X2A)] radical along with the methyl radical [CH3(X2A2′′)] and is open exclusively on the triplet surface with an overall reaction energy of −86 ± 10 kJ mol−1. The preferred reaction pathways proceed first by barrierless addition of triplet dicarbon to the π-electronic system of 1-butyne, either to both acetylenic carbon atoms or to the sterically more accessible carbon atom, to form the methyl-bearing triplet C6H6 intermediates [i41b] and [i81b], respectively, with the latter decomposing via a tight exit transition state to penta-1-yn-3,4-dienyl-1 [(H2CCCHCC(X2A)] plus the methyl radical [CH3(X2A2′′)]. The successful unraveling of this methyl-loss channel – through collaborative experimental and computational efforts – underscores the viability of the photolytically generated dicarbon beam as an unprecedented tool to access reaction dynamics underlying the formation of resonantly stabilized free radicals (RSFR) that are vital to molecular mass growth processes that ultimately lead to polycyclic aromatic hydrocarbons (PAHs).
中文翻译:

结合的交叉分子束和通过化学活化的C 5 H 4和C 6 H 6中间体形成独特的共振稳定的C 5 H 3自由基的计算研究†
交叉的分子束技术被利用到探索的共振稳定化(C的三种异构体形成5 ħ 3)基团与它们d沿2 -取代的对应物通过的单线/三重二碳[C的双分子反应2(X 1 Σ +克/ A 3 Π ù)]与甲基乙炔[CH 3 CCH(X 1阿1)],d 3 -methylacetylene [CD 3 CCH(X 1阿1)],和1-丁炔[C 2 H ^ 5 CCH(X 1通过化学活化的单重态/三重态C 5 H 4 / C 5 D 3 H和C 6 H 6中间体在碰撞能量高达26 kJ mol -1的情况下。这些研究利用了新开发的超音速二碳[C 2(X 1 Σ +克/ A 3 Π ù)]产生的光束经由四氯乙烯的光解[C 2氯4(X 1甲克)]排除了来自碳原子的干扰,碳原子表示基于消融的二碳源中的主要(干扰)物质。我们评估了二碳的性能[C 2(X 1 Σ +克/ A 3 Π ù)]束与甲基乙炔反应[CH 3 CCH(X 1阿1)]和d 3 -methylacetylene [CD 3 CCH(X 1 A 1)]; 研究表明,该反应动力学与我们实验室中使用基于消融的二碳源的合成方法有关,该方法涉及合成1,4-戊二炔基3 [HCCCHCCH(X 2 B1)]和2,4-戊二炔基-1 [H 2 CCCCCH(X 2 B 1)]自由基通过氢(氘)原子消除。考虑与c 2(X 1 Σ +克/ A 3 Π ù)-1-丁炔[C 2 H ^ 5 CCH(X 1 A')]反应,检测到迄今难以捉摸甲基损耗途径。这个通道形成先前未知的共振稳定化的戊-1-炔-3,4-二烯基-1- [H 2 CCCHCC(X 2 A)]与甲基基团一起自由基[CH 3(X 2甲2′')],并且仅在三重态表面上开放,总反应能量为-86±10 kJ mol -1。优选的反应途径首先通过将三重态二碳无阻地加到1-丁炔的π电子体系中,既加到炔属碳原子上,又加到空间上更易接近的碳原子上,形成含甲基的三重态C 6 H 6中间体。 [ i4 1b ]和[ i8 1b ]分别通过紧密的出口过渡态分解为五-1-基-3,4-二烯基-1 [(H 2 CCCHCC(X 2 A)]和甲基自由基[CH 3(X 2 A2 ′)]。通过合作的实验和计算工作,成功地阐明了这种甲基损失通道,突显了光解生成的二碳束的可行性,这是一种前所未有的工具,可用于获取对分子至关重要的共振稳定自由基(RSFR)形成基础的反应动力学质量增长过程,最终导致多环芳烃(PAH)。
更新日期:2018-03-08
中文翻译:

结合的交叉分子束和通过化学活化的C 5 H 4和C 6 H 6中间体形成独特的共振稳定的C 5 H 3自由基的计算研究†
交叉的分子束技术被利用到探索的共振稳定化(C的三种异构体形成5 ħ 3)基团与它们d沿2 -取代的对应物通过的单线/三重二碳[C的双分子反应2(X 1 Σ +克/ A 3 Π ù)]与甲基乙炔[CH 3 CCH(X 1阿1)],d 3 -methylacetylene [CD 3 CCH(X 1阿1)],和1-丁炔[C 2 H ^ 5 CCH(X 1通过化学活化的单重态/三重态C 5 H 4 / C 5 D 3 H和C 6 H 6中间体在碰撞能量高达26 kJ mol -1的情况下。这些研究利用了新开发的超音速二碳[C 2(X 1 Σ +克/ A 3 Π ù)]产生的光束经由四氯乙烯的光解[C 2氯4(X 1甲克)]排除了来自碳原子的干扰,碳原子表示基于消融的二碳源中的主要(干扰)物质。我们评估了二碳的性能[C 2(X 1 Σ +克/ A 3 Π ù)]束与甲基乙炔反应[CH 3 CCH(X 1阿1)]和d 3 -methylacetylene [CD 3 CCH(X 1 A 1)]; 研究表明,该反应动力学与我们实验室中使用基于消融的二碳源的合成方法有关,该方法涉及合成1,4-戊二炔基3 [HCCCHCCH(X 2 B1)]和2,4-戊二炔基-1 [H 2 CCCCCH(X 2 B 1)]自由基通过氢(氘)原子消除。考虑与c 2(X 1 Σ +克/ A 3 Π ù)-1-丁炔[C 2 H ^ 5 CCH(X 1 A')]反应,检测到迄今难以捉摸甲基损耗途径。这个通道形成先前未知的共振稳定化的戊-1-炔-3,4-二烯基-1- [H 2 CCCHCC(X 2 A)]与甲基基团一起自由基[CH 3(X 2甲2′')],并且仅在三重态表面上开放,总反应能量为-86±10 kJ mol -1。优选的反应途径首先通过将三重态二碳无阻地加到1-丁炔的π电子体系中,既加到炔属碳原子上,又加到空间上更易接近的碳原子上,形成含甲基的三重态C 6 H 6中间体。 [ i4 1b ]和[ i8 1b ]分别通过紧密的出口过渡态分解为五-1-基-3,4-二烯基-1 [(H 2 CCCHCC(X 2 A)]和甲基自由基[CH 3(X 2 A2 ′)]。通过合作的实验和计算工作,成功地阐明了这种甲基损失通道,突显了光解生成的二碳束的可行性,这是一种前所未有的工具,可用于获取对分子至关重要的共振稳定自由基(RSFR)形成基础的反应动力学质量增长过程,最终导致多环芳烃(PAH)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号