当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dissecting the Innate Immune Recognition of Opioid Inactive Isomer (+)-Naltrexone Derived Toll-like Receptor 4 (TLR4) Antagonists
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2018-03-08 00:00:00 , DOI: 10.1021/acs.jcim.7b00717 Xiaozheng Zhang 1, 2, 3 , Fengchao Cui 4 , Hongqian Chen 1 , Tianshu Zhang 1 , Kecheng Yang 2, 4 , Yibo Wang 1 , Zhenyan Jiang 5 , Kenner C. Rice 6 , Linda R. Watkins 7 , Mark R. Hutchinson 8 , Yunqi Li 4 , Yinghua Peng 9 , Xiaohui Wang 1
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2018-03-08 00:00:00 , DOI: 10.1021/acs.jcim.7b00717 Xiaozheng Zhang 1, 2, 3 , Fengchao Cui 4 , Hongqian Chen 1 , Tianshu Zhang 1 , Kecheng Yang 2, 4 , Yibo Wang 1 , Zhenyan Jiang 5 , Kenner C. Rice 6 , Linda R. Watkins 7 , Mark R. Hutchinson 8 , Yunqi Li 4 , Yinghua Peng 9 , Xiaohui Wang 1
Affiliation
|
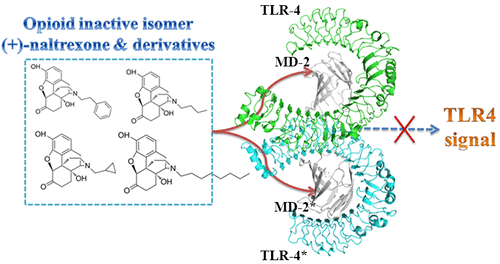
The opioid inactive isomer (+)-naltrexone is one of the rare Toll-like receptor 4 (TLR4) antagonists with good blood–brain barrier (BBB) permeability, which is a lead with promising potential for treating neuropathic pain and drug addiction. (+)-Naltrexone targets the lipopolysaccharides (LPS) binding pocket of myeloid differentiation protein 2 (MD-2) and blocks innate immune TLR4 signaling. However, the details of the molecular interactions of (+)-naltrexone and its derivatives with MD-2 are not fully understood, which hinders the ligand-based drug discovery. Herein, in silico and in vitro assays were performed to elucidate the innate immune recognition of the opioid inactive (+)-isomers. The results showed that the conserved LPS binding pocket of MD-2 accommodated these opioid inactive (+)-isomers. The calculated binding free energies of (+)-naltrexone and its derivatives in complex with MD-2 correlated well with their experimental binding affinities and TLR4 antagonistic activities. Hydrophobic residues in the MD-2 cavity interacted directly with these (+)-naltrexone based TLR4 antagonists and principally participated in ligand binding. Increasing the hydrophobicity of substituted group at N-17 improved its TLR4 antagonistic activity, while charged groups disfavored the binding with MD-2. Molecular dynamics (MD) simulations showed the binding of (+)-naltrexone or its derivatives to MD-2 stabilized the “collapsed” conformation of MD-2, consequently blocking the binding and signaling of TLR4. Thermodynamics and dynamic analysis showed the topology of substituted group at N-17 of (+)-naltrexone affected the binding with MD-2 and TLR4 antagonistic activity. This study provides a molecular insight into the innate immune recognition of opioid inactive (+)-isomers, which would be of great help for the development of next-generation of (+)-opioid based TLR4 antagonists.
中文翻译:

解剖阿片类药物非活性异构体(+)-纳曲酮衍生的Toll样受体4(TLR4)拮抗剂的先天免疫识别。
阿片类药物无活性异构体(+)-纳曲酮是一种罕见的Toll样受体4(TLR4)拮抗剂,具有良好的血脑屏障(BBB)渗透性,是治疗神经性疼痛和药物成瘾的潜在先导。(+)-纳曲酮靶向髓样分化蛋白2(MD-2)的脂多糖(LPS)结合口袋,并阻断先天免疫TLR4信号传导。然而,(+)-纳曲酮及其衍生物与MD-2的分子相互作用的细节尚未完全了解,这阻碍了基于配体的药物的发现。在本文中,在计算机上和体外进行测定以阐明阿片样物质无活性(+)-异构体的先天免疫识别。结果表明,保守的MD-2 LPS结合口袋容纳了这些阿片类药物的非活性(+)异构体。(+)-纳曲酮及其衍生物与MD-2复合的计算结合自由能与它们的实验结合亲和力和TLR4拮抗活性密切相关。MD-2空腔中的疏水残基直接与这些基于(+)-纳曲酮的TLR4拮抗剂相互作用,并且主要参与配体结合。增加取代基在N-17的疏水性可改善其TLR4拮抗活性,而带电基团则不利于与MD-2的结合。分子动力学(MD)模拟显示(+)-纳曲酮或其衍生物与MD-2的结合稳定了MD-2的“塌陷”构象,从而阻断了TLR4的结合和信号传导。热力学和动力学分析表明,(+)-纳曲酮N-17处取代基的拓扑结构影响了与MD-2和TLR4拮抗活性的结合。这项研究提供了对阿片类药物无活性(+)异构体的先天免疫识别的分子洞察力,这将对下一代基于(+)-阿片类药物的TLR4拮抗剂的开发有很大帮助。
更新日期:2018-03-08
中文翻译:

解剖阿片类药物非活性异构体(+)-纳曲酮衍生的Toll样受体4(TLR4)拮抗剂的先天免疫识别。
阿片类药物无活性异构体(+)-纳曲酮是一种罕见的Toll样受体4(TLR4)拮抗剂,具有良好的血脑屏障(BBB)渗透性,是治疗神经性疼痛和药物成瘾的潜在先导。(+)-纳曲酮靶向髓样分化蛋白2(MD-2)的脂多糖(LPS)结合口袋,并阻断先天免疫TLR4信号传导。然而,(+)-纳曲酮及其衍生物与MD-2的分子相互作用的细节尚未完全了解,这阻碍了基于配体的药物的发现。在本文中,在计算机上和体外进行测定以阐明阿片样物质无活性(+)-异构体的先天免疫识别。结果表明,保守的MD-2 LPS结合口袋容纳了这些阿片类药物的非活性(+)异构体。(+)-纳曲酮及其衍生物与MD-2复合的计算结合自由能与它们的实验结合亲和力和TLR4拮抗活性密切相关。MD-2空腔中的疏水残基直接与这些基于(+)-纳曲酮的TLR4拮抗剂相互作用,并且主要参与配体结合。增加取代基在N-17的疏水性可改善其TLR4拮抗活性,而带电基团则不利于与MD-2的结合。分子动力学(MD)模拟显示(+)-纳曲酮或其衍生物与MD-2的结合稳定了MD-2的“塌陷”构象,从而阻断了TLR4的结合和信号传导。热力学和动力学分析表明,(+)-纳曲酮N-17处取代基的拓扑结构影响了与MD-2和TLR4拮抗活性的结合。这项研究提供了对阿片类药物无活性(+)异构体的先天免疫识别的分子洞察力,这将对下一代基于(+)-阿片类药物的TLR4拮抗剂的开发有很大帮助。











































 京公网安备 11010802027423号
京公网安备 11010802027423号