当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tricarbonylrhenium(I) Complexes of Dinucleating Redox-Active Pincer Ligands
Organometallics ( IF 2.5 ) Pub Date : 2018-03-08 00:00:00 , DOI: 10.1021/acs.organomet.8b00013 James R. Gardinier 1 , Jeewantha S. Hewage 1 , Brian Bennett 2 , Denan Wang 1 , Sergey V. Lindeman 1
Organometallics ( IF 2.5 ) Pub Date : 2018-03-08 00:00:00 , DOI: 10.1021/acs.organomet.8b00013 James R. Gardinier 1 , Jeewantha S. Hewage 1 , Brian Bennett 2 , Denan Wang 1 , Sergey V. Lindeman 1
Affiliation
|
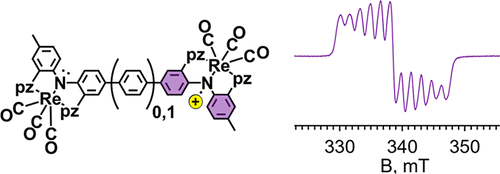
Two homobimetallic tricarbonylrhenium(I) complexes of new redox-active dinucleating pincer ligands have been prepared to assess the impact of spacer size on the first oxidation potentials with respect to mononucleating analogues and on intramolecular electronic communication. The new pincer ligands feature two tridentate NNN- sites each composed of two pyrazolyl flanking donors and a diarylamido anchor that are either directly linked (to form a central benzidene core, H2(L1)) or linked via a para-phenylene group (to form a para-terphenyldiamine core, H2(L2)). The bimetallic complexes of the deprotonated ligands, [fac-Re(CO)3]2(μ-L1), 1, and [fac-Re(CO)3]2(μ-L2), 2, were fully characterized in solution and the solid state including by single-crystal X-ray diffraction for 1. The electrochemical properties of each depended strongly on solvent and electrolyte. Complex 1 exhibits two one-electron oxidations in all electrolyte-containing solutions but with separations between first and second oxidation potentials, ΔE1/2, between 119 and 316 mV depending on conditions. On the other hand, cyclic voltammetry of 2 showed one two-electron oxidation in DMF with NBu4PF6 as an electrolyte but two one-electron oxidations with a maximal separation in ΔE1/2 of 96 mV in CH2Cl2 with NBu4B(C6F5)4 as an electrolyte. The oxidized complexes 1n+ and 2n+ (n = 1, 2) were prepared by chemical oxidation and were studied spectroscopically (UV–vis/NIR, EPR). The mono-oxidized complex 1+ behaves as a Robin–Day Class III species, while 2+ is a Robin–Day Class II species that shows thermal valence trapping at 77 K by EPR spectroscopy. As suggested from theoretical studies using DFT methods, the oxidized complexes maintain considerable ligand radical character, so their electronic structures can be formulated as (CO)3ReI(μ-Ln+)ReI(CO)3 (n = 1 or 2).
中文翻译:

双核氧化还原活性钳配体的三羰基hen(I)配合物
已经准备了两种新的具有氧化还原活性的双核夹钳配体的同双金属三羰基hen(I)络合物,以评估间隔物大小对单核类似物和分子内电子通讯对第一氧化电位的影响。新的钳形配体具有两个三齿NNN-位点,每个位点由两个吡唑基侧翼供体和一个二芳基酰胺锚定,这些位点可以直接连接(以形成中心亚苄基核心H 2(L1)),也可以通过对-亚苯基连接(以形成对-对苯二胺核心,H 2(L2))。去质子化配体[ fac -Re(CO)3 ] 2的双金属配合物(μ-L1),1,和[ FAC -Re(CO)3 ] 2(μ-L2),2,得到充分表征在溶液中并且包括由单晶X射线衍射用于固态1。每种的电化学性质在很大程度上取决于溶剂和电解质。配合物1在所有含电解质的溶液中均表现出两个单电子氧化,但取决于条件,第一和第二氧化电位之间的间隔ΔE 1/2在119至316 mV之间。另一方面,2的循环伏安法显示在NBu 4 PF 6的DMF中有一个双电子氧化。作为电解质,但在NBu 4 B(C 6 F 5)4作为CH 2 Cl 2中的两个单电子氧化中,最大分离距离为96 mV的ΔE 1/2。氧化的络合物1 n +和2 n +(n = 1,2)是通过化学氧化制备的,并进行了光谱学研究(UV-vis / NIR,EPR)。单氧化复合物1 +表现为Robin-Day III类,而2 +是一种Robin-II类物种,通过EPR光谱显示在77 K处有热价陷阱。如从使用DFT方法的理论研究表明,氧化复合物保持相当大的配体基团的字符,所以它们的电子结构可以被配制为(CO)3重新我(μ-L Ñ +)重新我(CO)3(ñ = 1或2)。
更新日期:2018-03-09
中文翻译:

双核氧化还原活性钳配体的三羰基hen(I)配合物
已经准备了两种新的具有氧化还原活性的双核夹钳配体的同双金属三羰基hen(I)络合物,以评估间隔物大小对单核类似物和分子内电子通讯对第一氧化电位的影响。新的钳形配体具有两个三齿NNN-位点,每个位点由两个吡唑基侧翼供体和一个二芳基酰胺锚定,这些位点可以直接连接(以形成中心亚苄基核心H 2(L1)),也可以通过对-亚苯基连接(以形成对-对苯二胺核心,H 2(L2))。去质子化配体[ fac -Re(CO)3 ] 2的双金属配合物(μ-L1),1,和[ FAC -Re(CO)3 ] 2(μ-L2),2,得到充分表征在溶液中并且包括由单晶X射线衍射用于固态1。每种的电化学性质在很大程度上取决于溶剂和电解质。配合物1在所有含电解质的溶液中均表现出两个单电子氧化,但取决于条件,第一和第二氧化电位之间的间隔ΔE 1/2在119至316 mV之间。另一方面,2的循环伏安法显示在NBu 4 PF 6的DMF中有一个双电子氧化。作为电解质,但在NBu 4 B(C 6 F 5)4作为CH 2 Cl 2中的两个单电子氧化中,最大分离距离为96 mV的ΔE 1/2。氧化的络合物1 n +和2 n +(n = 1,2)是通过化学氧化制备的,并进行了光谱学研究(UV-vis / NIR,EPR)。单氧化复合物1 +表现为Robin-Day III类,而2 +是一种Robin-II类物种,通过EPR光谱显示在77 K处有热价陷阱。如从使用DFT方法的理论研究表明,氧化复合物保持相当大的配体基团的字符,所以它们的电子结构可以被配制为(CO)3重新我(μ-L Ñ +)重新我(CO)3(ñ = 1或2)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号