Talanta ( IF 5.6 ) Pub Date : 2018-03-08 , DOI: 10.1016/j.talanta.2018.03.015 Elena Domínguez-Vega , Sara Tengattini , Claudia Peintner , Jordy van Angeren , Caterina Temporini , Rob Haselberg , Gabriella Massolini , Govert W. Somsen
|
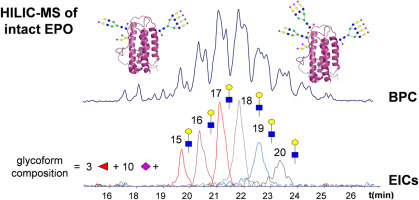
Glycosylation is considered a critical quality attribute of therapeutic proteins. Protein heterogeneity introduced by glycosylation includes differences in the nature, number and position of the glycans. Whereas analysis of released glycans and glycopeptides provides information about the composition and/or position of the glycan, intact glycoprotein analysis allows assignment of individual proteoforms and co-occurring modifications. Yet, resolving protein glycoforms at the intact level is challenging. We have explored the capacity of hydrophilic liquid chromatography-mass spectrometry (HILIC-MS) for assessing glycosylation patterns of intact pharmaceutical proteins by analyzing the complex glycoproteins interferon-beta-1a (rhIFN-β − 1a) and recombinant human erythropoietin (rhEPO). Efficient glycoform separation was achieved using a superficially-porous amide HILIC stationary phase and trifluoroacetic acid (TFA) as eluent additive. In-source collision-induced dissociation proved to be very useful to minimize protein-signal suppression effects by TFA. Direct injection of therapeutic proteins in aqueous formulation was possible without causing extra band dispersion, provided that the sample injection volume was not larger than 2 μL. HILIC-MS of rhIFN-β − 1a and rhEPO allowed the assignment of, respectively, 15 and 51 glycoform compositions, next to a variety of posttranslational modifications, such as succinimide, oxidation and N-terminal methionine-loss products. MS-based assignments showed that neutral glycan units significantly contributed to glycoform separation, whereas terminal sialic acids only had a marginal effect on HILIC retention. Comparisons of HILIC-MS with the selectivity provided by capillary electrophoresis-MS for the same glycoproteins, revealed a remarkable complementarity of the techniques. Finally it was demonstrated that by replacing TFA for difluoroacetic acid, peak resolution somewhat decreased, but rhEPO glycoforms with relative abundances below 1% could be detected by HILIC-MS, increasing the overall rhEPO glycoform coverage to 72.
中文翻译:

亲水相互作用色谱-质谱法对完整的治疗性蛋白质进行高分辨率糖型图谱分析
糖基化被认为是治疗性蛋白质的关键质量属性。通过糖基化引入的蛋白质异质性包括聚糖的性质,数量和位置的差异。释放的聚糖和糖肽的分析提供了有关聚糖的组成和/或位置的信息,而完整的糖蛋白分析则可以分配各个蛋白形式并同时发生修饰。然而,要完整地解析蛋白质糖型是具有挑战性的。我们已经通过分析复杂的糖蛋白干扰素-β-1a(rhIFN-β-1a)和重组人促红细胞生成素(rhEPO),探索了亲水液相色谱质谱(HILIC-MS)评估完整药物蛋白糖基化模式的能力。使用表面多孔酰胺HILIC固定相和三氟乙酸(TFA)作为洗脱液添加剂可实现有效的糖型分离。事实证明,源内碰撞诱导的解离对于最小化TFA抑制蛋白质信号的作用非常有用。只要样品进样体积不大于2μL,就可以在水性制剂中直接注射治疗性蛋白而不会引起额外的谱带分散。重组人IFN-β-1a和rhEPO的HILIC-MS可以分别分配15和51种糖型组合物,以及各种翻译后修饰,例如琥珀酰亚胺,氧化和N端甲硫氨酸丢失产物。基于质谱的研究表明,中性聚糖单元显着促进了糖型分离,而末端唾液酸仅对HILIC保留有边际影响。HILIC-MS与毛细管电泳-MS对相同糖蛋白的选择性的比较表明,该技术具有显着的互补性。最终证明,用TFA代替二氟乙酸,峰分离度有所降低,但HILIC-MS可以检测到相对丰度低于1%的rhEPO糖型,从而使总rhEPO糖型覆盖率提高到72。











































 京公网安备 11010802027423号
京公网安备 11010802027423号