Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
ATXN1-CIC Complex Is the Primary Driver of Cerebellar Pathology in Spinocerebellar Ataxia Type 1 through a Gain-of-Function Mechanism
Neuron ( IF 14.7 ) Pub Date : 2018-03-08 , DOI: 10.1016/j.neuron.2018.02.013 Maxime W C Rousseaux 1 , Tyler Tschumperlin 2 , Hsiang-Chih Lu 3 , Elizabeth P Lackey 4 , Vitaliy V Bondar 1 , Ying-Wooi Wan 1 , Qiumin Tan 1 , Carolyn J Adamski 1 , Jillian Friedrich 2 , Kirk Twaroski 5 , Weili Chen 5 , Jakub Tolar 5 , Christine Henzler 6 , Ajay Sharma 1 , Aleksandar Bajić 1 , Tao Lin 7 , Lisa Duvick 2 , Zhandong Liu 8 , Roy V Sillitoe 4 , Huda Y Zoghbi 9 , Harry T Orr 2
Neuron ( IF 14.7 ) Pub Date : 2018-03-08 , DOI: 10.1016/j.neuron.2018.02.013 Maxime W C Rousseaux 1 , Tyler Tschumperlin 2 , Hsiang-Chih Lu 3 , Elizabeth P Lackey 4 , Vitaliy V Bondar 1 , Ying-Wooi Wan 1 , Qiumin Tan 1 , Carolyn J Adamski 1 , Jillian Friedrich 2 , Kirk Twaroski 5 , Weili Chen 5 , Jakub Tolar 5 , Christine Henzler 6 , Ajay Sharma 1 , Aleksandar Bajić 1 , Tao Lin 7 , Lisa Duvick 2 , Zhandong Liu 8 , Roy V Sillitoe 4 , Huda Y Zoghbi 9 , Harry T Orr 2
Affiliation
|
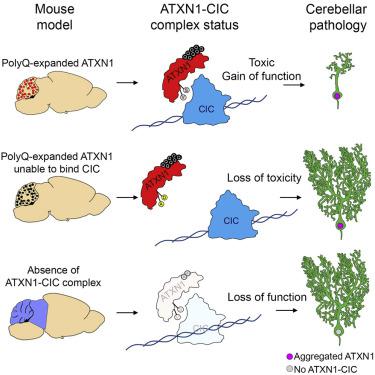
Polyglutamine (polyQ) diseases are caused by expansion of translated CAG repeats in distinct genes leading to altered protein function. In spinocerebellar ataxia type 1 (SCA1), a gain of function of polyQ-expanded ataxin-1 (ATXN1) contributes to cerebellar pathology. The extent to which cerebellar toxicity depends on its cognate partner capicua (CIC), versus other interactors, remains unclear. It is also not established whether loss of the ATXN1-CIC complex in the cerebellum contributes to disease pathogenesis. In this study, we exclusively disrupt the ATXN1-CIC interaction and show that it is at the crux of cerebellar toxicity in SCA1. Importantly, loss of CIC in the cerebellum does not cause ataxia or Purkinje cell degeneration. Expression profiling of these gain- and loss-of-function models, coupled with data from iPSC-derived neurons from SCA1 patients, supports a mechanism in which gain of function of the ATXN1-CIC complex is the major driver of toxicity.
中文翻译:

ATXN1-CIC 复合体通过功能获得机制是 1 型脊髓小脑共济失调小脑病理学的主要驱动因素
多聚谷氨酰胺 (polyQ) 疾病是由不同基因中翻译的 CAG 重复序列扩展导致蛋白质功能改变引起的。在 1 型脊髓小脑共济失调 (SCA1) 中,polyQ 扩展的 ataxin-1 (ATXN1) 的功能增强会导致小脑病理学。小脑毒性在多大程度上取决于其同源伴侣 capicua (CIC) 与其他相互作用因素,目前尚不清楚。小脑中 ATXN1-CIC 复合物的缺失是否会导致疾病的发病机制也尚未确定。在这项研究中,我们专门破坏了 ATXN1-CIC 相互作用,并表明它是 SCA1 中小脑毒性的关键。重要的是,小脑中 CIC 的缺失不会导致共济失调或浦肯野细胞变性。这些功能获得和丧失模型的表达谱,加上来自 SCA1 患者的 iPSC 衍生神经元的数据,支持了一种机制,其中 ATXN1-CIC 复合物的功能获得是毒性的主要驱动因素。
更新日期:2018-03-08
中文翻译:

ATXN1-CIC 复合体通过功能获得机制是 1 型脊髓小脑共济失调小脑病理学的主要驱动因素
多聚谷氨酰胺 (polyQ) 疾病是由不同基因中翻译的 CAG 重复序列扩展导致蛋白质功能改变引起的。在 1 型脊髓小脑共济失调 (SCA1) 中,polyQ 扩展的 ataxin-1 (ATXN1) 的功能增强会导致小脑病理学。小脑毒性在多大程度上取决于其同源伴侣 capicua (CIC) 与其他相互作用因素,目前尚不清楚。小脑中 ATXN1-CIC 复合物的缺失是否会导致疾病的发病机制也尚未确定。在这项研究中,我们专门破坏了 ATXN1-CIC 相互作用,并表明它是 SCA1 中小脑毒性的关键。重要的是,小脑中 CIC 的缺失不会导致共济失调或浦肯野细胞变性。这些功能获得和丧失模型的表达谱,加上来自 SCA1 患者的 iPSC 衍生神经元的数据,支持了一种机制,其中 ATXN1-CIC 复合物的功能获得是毒性的主要驱动因素。









































 京公网安备 11010802027423号
京公网安备 11010802027423号