Journal of Controlled Release ( IF 10.5 ) Pub Date : 2018-03-09 , DOI: 10.1016/j.jconrel.2018.03.002 Lucía Martínez-Jothar , Sofia Doulkeridou , Raymond M. Schiffelers , Javier Sastre Torano , Sabrina Oliveira , Cornelus F. van Nostrum , Wim E. Hennink
|
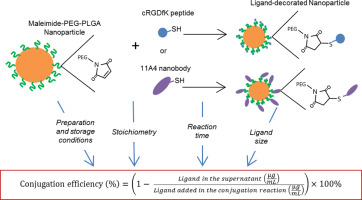
Maleimide-thiol chemistry is widely used for the design and preparation of ligand-decorated drug delivery systems such as poly(lactide-co-glycolide) (PLGA) based nanoparticles (NPs). While many publications on nanocarriers functionalized exploiting this strategy are available in the literature, the conditions at which this reaction takes place vary among publications. This paper presents a comprehensive study on the conjugation of the peptide cRGDfK and the nanobody 11A4 (both containing a free thiol group) to maleimide functionalized PLGA NPs by means of the maleimide-thiol click reaction. The influence of different parameters, such as the nanoparticles preparation method and storage conditions as well as the molar ratio of maleimide to ligand used for conjugation, on the reaction efficiency has been evaluated. The NPs were prepared by a single or double emulsion method using different types and concentrations of surfactants and stored at 4 or 20 °C before reaction with the targeting moieties. Several maleimide to ligand molar ratios and different reaction times were studied and the conjugation efficiency was determined by quantification of the not-bound ligand by liquid chromatography. The kind of emulsion used to prepare the NPs as well as the type and concentration of surfactant used had no effect on the conjugation efficiency. Reaction between the maleimide groups present in the NPs and cRGDfK was optimal at a maleimide to thiol molar ratio of 2:1, reaching a conjugation efficiency of 84 ± 4% after 30 min at room temperature in 10 mM HEPES pH 7.0. For 11A4 nanobody the optimal reaction efficiency, 58 ± 12%, was achieved after 2 h of incubation at room temperature in PBS pH 7.4 using a 5:1 maleimide to protein molar ratio. Storage of the NPs at 4 °C for 7 days prior to their exposure to the ligands resulted in approximately 10% decrease in the reactivity of maleimide in contrast to storage at 20 °C which led to almost 40% of the maleimide being unreactive after the same storage time. Our findings demonstrate that optimization of this reaction, particularly in terms of reactant ratios, can represent a significant increase in the conjugation efficiency and prevent considerable waste of resources.
中文翻译:

对马来酰亚胺-硫醇共轭化学的见解:用于受体靶向的纳米颗粒有效表面功能化的条件
马来酰亚胺-硫醇化学被广泛地用于配体的装饰的药物递送系统,例如聚的设计和制备(丙交酯-共-乙交酯)(PLGA)基纳米颗粒(NPs)。尽管文献中提供了许多有关利用这种策略功能化的纳米载体的出版物,但该反应发生的条件因出版物而异。本文介绍了通过马来酰亚胺-硫醇点击反应将肽cRGDfK和纳米抗体11A4(均含有游离硫醇基)与马来酰亚胺官能化的PLGA NP偶联的综合研究。评估了不同参数(例如纳米颗粒的制备方法和储存条件以及用于共轭的马来酰亚胺与配体的摩尔比)对反应效率的影响。通过使用不同类型和浓度的表面活性剂通过单乳液或双乳液方法制备NP,并在与目标部分反应之前将其存储在4或20°C下。研究了几种马来酰亚胺与配体的摩尔比和不同的反应时间,并通过液相色谱定量未结合的配体来确定缀合效率。用于制备NP的乳液的种类以及所使用的表面活性剂的类型和浓度对缀合效率没有影响。NP中存在的马来酰亚胺基团与cRGDfK之间的反应在马来酰亚胺与硫醇的摩尔比为2:1时是最佳的,室温下在30 m下于10 mM HEPES pH 7.0中达到的缀合效率为84±4%。对于11A4纳米抗体,最佳反应效率为58±12%,在室温下于5:1马来酰亚胺与蛋白质的摩尔比的PBS pH 7.4中温育2小时后,获得了λ。NP在暴露于配体之前在4°C下保存7天,导致马来酰亚胺的反应性降低约10%,而在20°C下保存则导致几乎40%的马来酰亚胺在反应后不反应。相同的存储时间。我们的发现表明,该反应的优化,尤其是在反应物比例方面的优化,可以代表共轭效率的显着提高,并防止大量的资源浪费。NP在暴露于配体之前在4°C下保存7天,导致马来酰亚胺的反应性降低约10%,而在20°C下保存则导致几乎40%的马来酰亚胺在反应后不反应。相同的存储时间。我们的发现表明,该反应的优化,尤其是在反应物比例方面的优化,可以代表共轭效率的显着提高,并防止大量的资源浪费。NP在暴露于配体之前在4°C下保存7天,导致马来酰亚胺的反应性降低约10%,而在20°C下保存则导致几乎40%的马来酰亚胺在反应后不反应。相同的存储时间。我们的发现表明,该反应的优化,尤其是在反应物比例方面的优化,可以代表共轭效率的显着提高,并防止大量的资源浪费。











































 京公网安备 11010802027423号
京公网安备 11010802027423号