当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical Studies of the Formation Mechanisms, Thermodynamic Stabilities, and Water-Exchange Reactivities of Aluminum-Salicylate Complexes in Aqueous Solution
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2018-03-08 00:00:00 , DOI: 10.1021/acsearthspacechem.7b00141 Shaonan Dong 1 , Wenjing Shi 1 , Jing Zhang 1 , Shuping Bi 1
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2018-03-08 00:00:00 , DOI: 10.1021/acsearthspacechem.7b00141 Shaonan Dong 1 , Wenjing Shi 1 , Jing Zhang 1 , Shuping Bi 1
Affiliation
|
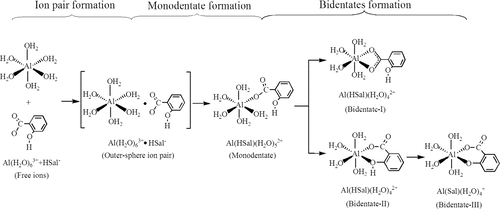
The formation mechanisms, thermodynamic stabilities, and water-exchange reactivities of 1:1 monomer aluminum–salicylate (Al–salicylate) complexes in acidic aqueous solution are investigated using the density functional theory-quantum chemical cluster model (DFT-CM) method. (1) The formation pathways for possible monodentate and bidentate Al–salicylate configurations are modeled with the gas phase-supermolecule-polarizable continuum model (GP-SM-PCM). It shows that the formation pathways for the Al–salicylate complexes follow the Eigen-Wilkins mechanism, where the dissociation of an inner-shell coordinated water of Al3+ is the rate-determining step. (2) The formation constants Kaq for different Al–salicylate configurations are estimated based on the total Gibbs free energy changes ΔG° for their overall formation pathways. It is indicated that in the acidic aqueous solution at pH ∼ 3, the main existence form of the 1:1 monomer Al–salicylate complex is the phenol-deprotonated bidentate Al(Sal)(H2O)4+ with six-membered ring. Its log Kaq is calculated as 13.8, in good agreement with the literature values of 12.9–14.5. (3) The water-exchange reactions are modeled for different Al–salicylate configurations. The water-exchange rate constant for Al(Sal)(H2O)4+ is estimated as log kH2O = 3.9 s–1, close to the experimental value of 3.7 s–1. It proves again that this configuration is the dominant form under experimental conditions.
中文翻译:

水杨酸铝络合物在水溶液中的形成机理,热力学稳定性和水交换反应性的理论研究
使用密度泛函理论-量子化学簇模型(DFT-CM)方法研究了酸性水溶液中1:1单体水杨酸铝(Al-水杨酸)配合物的形成机理,热力学稳定性和水交换反应性。(1)用气相-超分子可极化连续体模型(GP-SM-PCM)对可能的单齿和双齿铝水杨酸酯构型的形成途径进行建模。它表明,铝水杨酸酯复合物的形成途径遵循本征-维金斯机理,其中Al 3+的内壳配位水的解离是决定速率的步骤。(2)地层常数K aq根据其整个形成途径的总吉布斯自由能变化ΔG °估算不同的水杨酸铝构型。结果表明,在pH〜3的酸性水溶液中,1:1单体水杨酸配合物的主要存在形式是具有六元环的苯酚脱质子化双齿铝(Sal)(H 2 O)4 +。 。计算得出其log K aq为13.8,与文献值12.9–14.5很好地吻合。(3)对水交换反应建模为不同的水杨酸铝构型。Al(Sal)(H 2 O)4 +的水交换速率常数估计为log k H2O= 3.9 s –1,接近3.7 s –1的实验值。再次证明了该构型是实验条件下的主要形式。
更新日期:2018-03-08
中文翻译:

水杨酸铝络合物在水溶液中的形成机理,热力学稳定性和水交换反应性的理论研究
使用密度泛函理论-量子化学簇模型(DFT-CM)方法研究了酸性水溶液中1:1单体水杨酸铝(Al-水杨酸)配合物的形成机理,热力学稳定性和水交换反应性。(1)用气相-超分子可极化连续体模型(GP-SM-PCM)对可能的单齿和双齿铝水杨酸酯构型的形成途径进行建模。它表明,铝水杨酸酯复合物的形成途径遵循本征-维金斯机理,其中Al 3+的内壳配位水的解离是决定速率的步骤。(2)地层常数K aq根据其整个形成途径的总吉布斯自由能变化ΔG °估算不同的水杨酸铝构型。结果表明,在pH〜3的酸性水溶液中,1:1单体水杨酸配合物的主要存在形式是具有六元环的苯酚脱质子化双齿铝(Sal)(H 2 O)4 +。 。计算得出其log K aq为13.8,与文献值12.9–14.5很好地吻合。(3)对水交换反应建模为不同的水杨酸铝构型。Al(Sal)(H 2 O)4 +的水交换速率常数估计为log k H2O= 3.9 s –1,接近3.7 s –1的实验值。再次证明了该构型是实验条件下的主要形式。









































 京公网安备 11010802027423号
京公网安备 11010802027423号