Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2018-03-08 , DOI: 10.1016/j.bioorg.2018.03.010 Charles H Clapp 1 , Justin Pachuski 1 , Natasha F Bassett 1 , Kathleen A Bishop 1 , Gillian Carter 1 , Megan Young 1 , Thomas Young 1 , Yuhan Fu 1
|
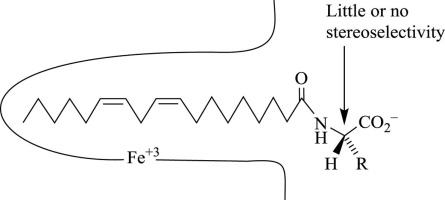
Lipoxygenases catalyze the oxygenation of polyunsaturated fatty acids and their derivatives to produce conjugated diene hydroperoxides. Soybean lipoxygenase-1 (SBLO-1) has been the subject of intensive structural and mechanistic study, but the manner in which this enzyme binds substrates is uncertain. Previous studies suggest that the fatty acyl group of the substrate binds in an internal cavity near the catalytic iron with the polar end at the surface of the protein or perhaps external to the protein. To test this model, we have investigated two pairs of enantiomeric N-linoleoylamino acids as substrates for SBLO-1. If the amino acid moiety binds external to the protein, the kinetics and product distribution should show little or no sensitivity to the stereochemical configuration of the amino acid moiety. Consistent with this expectation, N-linoleoyl-l-valine (LLV) and N-linoleoyl-d-valine (LDV) are both good substrates with kcat/Km values that are equal within error and about 40% higher than kcat/Km for linoleic acid. Experiments with N-linoleoyl-l-tryptophan (LLT) and N-linoleoyl-d-tryptophan (LDT) were complicated by the low critical micelle concentrations (CMC = 6–8 μM) of these substances. Below the CMC, LDT is a better substrate by a factor of 2.7. The rates of oxygenation of LDT and LLT continue to rise above the CMC, with modest stereoselectivity in favor of the d enantiomer. With all of the substrates tested, the major product is the 13(S)-hydroperoxide, and the distribution of minor products is not appreciably affected by the configuration of the amino acid moiety. The absence of stereoselectivity with LLV and LDV, the modest magnitude of the stereoselectivity with LLT and LDT, and the ability micellar forms of LLT and LDT to increase the concentration of available substrate are all consistent with the hypothesis that the amino acid moiety binds largely external to SBLO-1 and interacts with it only weakly.
中文翻译:

N-亚油酰氨基酸作为大豆脂氧合酶-1 底物结合的手性探针
脂加氧酶催化多不饱和脂肪酸及其衍生物的氧化作用,产生共轭二烯氢过氧化物。大豆脂氧合酶-1 (SBLO-1) 一直是深入的结构和机制研究的主题,但该酶与底物结合的方式尚不确定。先前的研究表明,底物的脂肪酰基在催化铁附近的内部空腔中结合,极性端位于蛋白质表面或可能在蛋白质外部。为了测试这个模型,我们研究了两对对映体 N-亚油酰氨基酸作为 SBLO-1 的底物。如果氨基酸部分结合在蛋白质外部,则动力学和产物分布应该对氨基酸部分的立体化学构型表现出很少或没有敏感性。与这一预期一致,N-亚油酰-l-缬氨酸 (LLV) 和 N-亚油酰-d-缬氨酸 ( LDV ) 都是良好的底物,其 k cat /K m值在误差范围内相等,并且比 k cat高约 40% /K m为亚油酸。 N-亚油酰-l-色氨酸 (LLT) 和 N-亚油酰-d-色氨酸 (LDT) 的实验因这些物质的低临界胶束浓度 (CMC = 6–8 μM) 而变得复杂。低于 CMC 时,LDT 是更好的基材,其性能好 2.7 倍。 LDT 和 LLT 的氧合速率继续上升至高于 CMC,并具有有利于反映异构体的适度立体选择性。对于所有测试的底物,主要产物是13( S) -氢过氧化物,次要产物的分布不受氨基酸部分构型的明显影响。 LLV 和 LDV 不存在立体选择性,LLT 和 LDT 立体选择性适度,以及 LLT 和 LDT 的胶束形式增加可用底物浓度的能力都与氨基酸部分主要结合外部的假设一致。 SBLO-1 并与其仅微弱相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号