当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of 2,6,7-Trisubstituted Prenylated indole
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-03-08 00:00:00 , DOI: 10.1021/acs.joc.7b03273 Motoki Shiozawa 1 , Keisuke Iida 2 , Minami Odagi 1 , Masahiro Yamanaka 3 , Kazuo Nagasawa 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-03-08 00:00:00 , DOI: 10.1021/acs.joc.7b03273 Motoki Shiozawa 1 , Keisuke Iida 2 , Minami Odagi 1 , Masahiro Yamanaka 3 , Kazuo Nagasawa 1
Affiliation
|
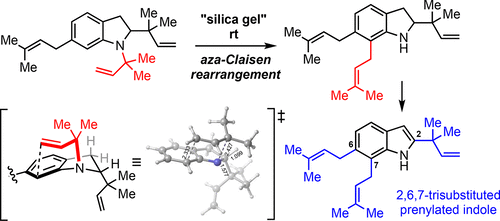
Prenylated indole alkaloids bearing more than one prenyl or reverse-prenyl group show various biological activities. Among them, synthesis of trisubstituted-type prenylated indoles have not been well explored because of the difficulty in regioselective introduction of multiple prenyl and reverse-prenyl groups due to steric hindrance problems. Herein, we describe a synthesis of 2,6,7-trisubstituted prenylated indole using aza-Claisen rearrangement under mild conditions to introduce a prenyl group at C7 in the presence of the prenyl group at C6.
中文翻译:

2,6,7-三取代的炔丙基吲哚的合成
带有一个以上异戊烯基或反向异戊烯基的炔丙基吲哚生物碱具有多种生物活性。其中,由于空间位阻问题,由于难以将多个异戊烯基和反异戊烯基基团进行区域选择性引入,所以尚未很好地探索三取代型炔丙基化吲哚的合成。在本文中,我们描述了在温和条件下使用氮杂-克莱森重排在C6异戊烯基存在的情况下在C7引入异戊二烯基的合成2,6,7-三取代的炔丙基吲哚的方法。
更新日期:2018-03-08
中文翻译:

2,6,7-三取代的炔丙基吲哚的合成
带有一个以上异戊烯基或反向异戊烯基的炔丙基吲哚生物碱具有多种生物活性。其中,由于空间位阻问题,由于难以将多个异戊烯基和反异戊烯基基团进行区域选择性引入,所以尚未很好地探索三取代型炔丙基化吲哚的合成。在本文中,我们描述了在温和条件下使用氮杂-克莱森重排在C6异戊烯基存在的情况下在C7引入异戊二烯基的合成2,6,7-三取代的炔丙基吲哚的方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号