当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Role of Ligand-Driven Conformational Changes in Enzyme Catalysis: Modeling the Reactivity of the Catalytic Cage of Triosephosphate Isomerase
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-03-08 , DOI: 10.1021/jacs.8b00251 Yashraj S Kulkarni 1 , Qinghua Liao 1 , Fabian Byléhn 1, 2 , Tina L Amyes 3 , John P Richard 3 , Shina C L Kamerlin 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-03-08 , DOI: 10.1021/jacs.8b00251 Yashraj S Kulkarni 1 , Qinghua Liao 1 , Fabian Byléhn 1, 2 , Tina L Amyes 3 , John P Richard 3 , Shina C L Kamerlin 1
Affiliation
|
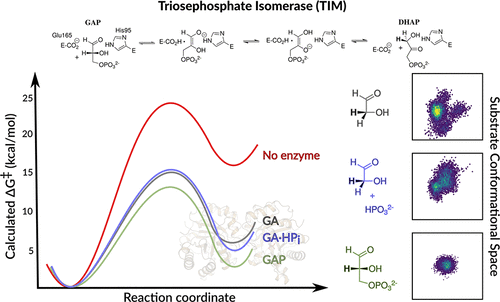
We have previously performed empirical valence bond calculations of the kinetic activation barriers, ΔG‡calc, for the deprotonation of complexes between TIM and the whole substrate glyceraldehyde-3-phosphate (GAP, Kulkarni et al.J. Am. Chem. Soc.2017, 139, 10514–1052528683550). We now extend this work to also study the deprotonation of the substrate pieces glycolaldehyde (GA) and GA·HPi [HPi = phosphite dianion]. Our combined calculations provide activation barriers, ΔG‡calc, for the TIM-catalyzed deprotonation of GAP (12.9 ± 0.8 kcal·mol–1), of the substrate piece GA (15.0 ± 2.4 kcal·mol–1), and of the pieces GA·HPi (15.5 ± 3.5 kcal·mol–1). The effect of bound dianion on ΔG‡calc is small (≤2.6 kcal·mol–1), in comparison to the much larger 12.0 and 5.8 kcal·mol–1 intrinsic phosphodianion and phosphite dianion binding energy utilized to stabilize the transition states for TIM-catalyzed deprotonation of GAP and GA·HPi, respectively. This shows that the dianion binding energy is essentially fully expressed at our protein model for the Michaelis complex, where it is utilized to drive an activating change in enzyme conformation. The results represent an example of the synergistic use of results from experiments and calculations to advance our understanding of enzymatic reaction mechanisms.
中文翻译:

配体驱动的构象变化在酶催化中的作用:模拟磷酸丙糖异构酶催化笼的反应性
我们之前已经对动力学激活势垒 ΔG‡calc 进行了经验价键计算,用于 TIM 和整个底物 3-磷酸甘油醛之间的复合物的去质子化(GAP,Kulkarni 等人,J. Am. Chem. Soc.2017 , 139, 10514–1052528683550)。我们现在将这项工作扩展到还研究底物乙醇醛 (GA) 和 GA·HPi [HPi = 亚磷酸二价阴离子] 的去质子化。我们的综合计算为 TIM 催化的 GAP (12.9 ± 0.8 kcal·mol–1)、基底片 GA (15.0 ± 2.4 kcal·mol–1) 和片的去质子化提供了激活势垒ΔG‡calc GA·HPi (15.5 ± 3.5 kcal·mol–1)。与更大的 12.0 和 5 相比,结合二价阴离子对 ΔG‡calc 的影响很小(≤2.6 kcal·mol–1)。8 kcal·mol-1 本征磷酸二价阴离子和亚磷酸酯二价阴离子结合能分别用于稳定 TIM 催化的 GAP 和 GA·HPi 去质子化的过渡态。这表明二价阴离子结合能在我们的 Michaelis 复合物蛋白质模型中基本上完全表达,在那里它被用来驱动酶构象的激活变化。结果代表了协同使用实验和计算结果以促进我们对酶促反应机制的理解的一个例子。
更新日期:2018-03-08
中文翻译:

配体驱动的构象变化在酶催化中的作用:模拟磷酸丙糖异构酶催化笼的反应性
我们之前已经对动力学激活势垒 ΔG‡calc 进行了经验价键计算,用于 TIM 和整个底物 3-磷酸甘油醛之间的复合物的去质子化(GAP,Kulkarni 等人,J. Am. Chem. Soc.2017 , 139, 10514–1052528683550)。我们现在将这项工作扩展到还研究底物乙醇醛 (GA) 和 GA·HPi [HPi = 亚磷酸二价阴离子] 的去质子化。我们的综合计算为 TIM 催化的 GAP (12.9 ± 0.8 kcal·mol–1)、基底片 GA (15.0 ± 2.4 kcal·mol–1) 和片的去质子化提供了激活势垒ΔG‡calc GA·HPi (15.5 ± 3.5 kcal·mol–1)。与更大的 12.0 和 5 相比,结合二价阴离子对 ΔG‡calc 的影响很小(≤2.6 kcal·mol–1)。8 kcal·mol-1 本征磷酸二价阴离子和亚磷酸酯二价阴离子结合能分别用于稳定 TIM 催化的 GAP 和 GA·HPi 去质子化的过渡态。这表明二价阴离子结合能在我们的 Michaelis 复合物蛋白质模型中基本上完全表达,在那里它被用来驱动酶构象的激活变化。结果代表了协同使用实验和计算结果以促进我们对酶促反应机制的理解的一个例子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号