当前位置:
X-MOL 学术
›
Electroanalysis
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Preparation of Gold Modified Nickel Wire Electrodes for Electroanalysis via a Galvanic Replacement Reaction
Electroanalysis ( IF 2.7 ) Pub Date : 2018-03-08 , DOI: 10.1002/elan.201800077 Yuki Umeya 1 , Yusuke Kobayashi 1 , Toshiyuki Kawashimo 1 , Sunyhik Ahn 1 , Gang Chang 2 , Munetaka Oyama 1
Electroanalysis ( IF 2.7 ) Pub Date : 2018-03-08 , DOI: 10.1002/elan.201800077 Yuki Umeya 1 , Yusuke Kobayashi 1 , Toshiyuki Kawashimo 1 , Sunyhik Ahn 1 , Gang Chang 2 , Munetaka Oyama 1
Affiliation
|
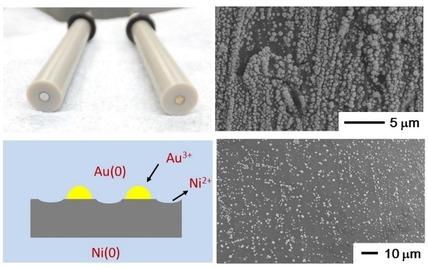
Gold modified nickel (Ni) electrodes were prepared via a simple galvanic replacement reaction between chloroauric acid (AuCl4−) and Ni base materials. Au nano- or micro-structures were deposited on the Ni surface by immersing Ni disk/wire electrode in an aqueous solution of AuCl4−. The concentration of AuCl4− and the immersion time were systematically varied, and the surfaces of Ni wire were characterized by scanning electron microscopy (SEM). Furthermore, electrochemical responses of ferrocyanide, uric acid and glucose were observed with Au modified Ni wire electrodes prepared at lower concentrations of AuCl4− (below 1.0×10−4 M) after fixing some conditions. Consequently, reversible electrochemical responses of ferrocyanide could be observed even for Ni wire treated with 1.0×10−6 M AuCl4−, though no deposition was observed in the SEM measurements. Also, control of electrocatalytic activity was possible below 1.0×10−4 M AuCl4− for uric acid and glucose. Judging from the tunable electrocatalytic performance, simplicity of preparation, and the cost of electrode materials, we believe that Au modified Ni electrodes holds promise for applications in electroanalysis. On the other hand, in our preliminary trials, the properties of deposited Au nano- or micro-particles were found to be sensitive to several factors. Further work is needed to achieve quantitative Au deposition over individual electrodes.
中文翻译:

通过电置换反应制备用于电分析的金改性镍丝电极
金改性镍 (Ni) 电极是通过氯金酸 (AuCl4−) 和镍基材料之间的简单电置换反应制备的。通过将 Ni 盘/线电极浸入 AuCl4- 水溶液中,Au 纳米或微结构沉积在 Ni 表面上。AuCl4− 的浓度和浸渍时间有系统地变化,Ni 线的表面通过扫描电子显微镜 (SEM) 进行表征。此外,在固定一些条件后,在较低浓度的 AuCl4-(低于 1.0×10-4 M)下制备的 Au 改性镍线电极观察到亚铁氰化物、尿酸和葡萄糖的电化学响应。因此,即使对于用 1.0×10-6 M AuCl4- 处理的 Ni 线,也可以观察到亚铁氰化物的可逆电化学响应,尽管在 SEM 测量中没有观察到沉积。此外,对于尿酸和葡萄糖,电催化活性的控制可能低于 1.0×10-4 M AuCl4-。从可调节的电催化性能、制备的简单性和电极材料的成本来看,我们认为 Au 修饰的 Ni 电极在电分析中的应用前景广阔。另一方面,在我们的初步试验中,发现沉积的 Au 纳米颗粒或微米颗粒的特性对几个因素很敏感。需要进一步的工作来在单个电极上实现定量的 Au 沉积。我们相信 Au 修饰的 Ni 电极在电解分析中的应用前景广阔。另一方面,在我们的初步试验中,发现沉积的 Au 纳米颗粒或微米颗粒的特性对几个因素很敏感。需要进一步的工作来在单个电极上实现定量的 Au 沉积。我们相信 Au 修饰的 Ni 电极在电解分析中的应用前景广阔。另一方面,在我们的初步试验中,发现沉积的 Au 纳米颗粒或微米颗粒的特性对几个因素很敏感。需要进一步的工作来在单个电极上实现定量的 Au 沉积。
更新日期:2018-03-08
中文翻译:

通过电置换反应制备用于电分析的金改性镍丝电极
金改性镍 (Ni) 电极是通过氯金酸 (AuCl4−) 和镍基材料之间的简单电置换反应制备的。通过将 Ni 盘/线电极浸入 AuCl4- 水溶液中,Au 纳米或微结构沉积在 Ni 表面上。AuCl4− 的浓度和浸渍时间有系统地变化,Ni 线的表面通过扫描电子显微镜 (SEM) 进行表征。此外,在固定一些条件后,在较低浓度的 AuCl4-(低于 1.0×10-4 M)下制备的 Au 改性镍线电极观察到亚铁氰化物、尿酸和葡萄糖的电化学响应。因此,即使对于用 1.0×10-6 M AuCl4- 处理的 Ni 线,也可以观察到亚铁氰化物的可逆电化学响应,尽管在 SEM 测量中没有观察到沉积。此外,对于尿酸和葡萄糖,电催化活性的控制可能低于 1.0×10-4 M AuCl4-。从可调节的电催化性能、制备的简单性和电极材料的成本来看,我们认为 Au 修饰的 Ni 电极在电分析中的应用前景广阔。另一方面,在我们的初步试验中,发现沉积的 Au 纳米颗粒或微米颗粒的特性对几个因素很敏感。需要进一步的工作来在单个电极上实现定量的 Au 沉积。我们相信 Au 修饰的 Ni 电极在电解分析中的应用前景广阔。另一方面,在我们的初步试验中,发现沉积的 Au 纳米颗粒或微米颗粒的特性对几个因素很敏感。需要进一步的工作来在单个电极上实现定量的 Au 沉积。我们相信 Au 修饰的 Ni 电极在电解分析中的应用前景广阔。另一方面,在我们的初步试验中,发现沉积的 Au 纳米颗粒或微米颗粒的特性对几个因素很敏感。需要进一步的工作来在单个电极上实现定量的 Au 沉积。











































 京公网安备 11010802027423号
京公网安备 11010802027423号