当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient access to chiral benzo[c]chromenes via asymmetric transfer hydrogenation of ketals†
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-03-08 00:00:00 , DOI: 10.1039/c8qo00096d Yangshan Li 1, 2, 3, 4 , Miao Wan 4, 5, 6, 7 , Shutao Sun 4, 5, 6, 7 , Zhongjun Fu 1, 2, 3, 4 , Haofei Huang 1, 2, 3, 4 , Lei Liu 1, 4, 5, 6, 7
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-03-08 00:00:00 , DOI: 10.1039/c8qo00096d Yangshan Li 1, 2, 3, 4 , Miao Wan 4, 5, 6, 7 , Shutao Sun 4, 5, 6, 7 , Zhongjun Fu 1, 2, 3, 4 , Haofei Huang 1, 2, 3, 4 , Lei Liu 1, 4, 5, 6, 7
Affiliation
|
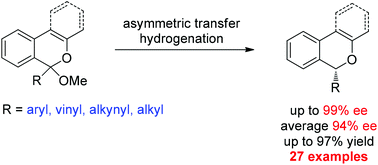
A highly enantioselective synthetic method to access chiral α-substituted 6H-benzo[c]chromenes through chiral imidodiphosphoric acid-catalyzed asymmetric transfer hydrogenation of the corresponding ketals has been established. The substituent patterns at the α-position proved to be unsusceptible to the asymmetric catalysis system, with diverse hybrid substituents including aryl and vinyl, alkynyl, and alkyl moieties that were well tolerated. In addition, the generality of the method was further demonstrated by application to 1H-isochromene-based ketals, offering chiral α-aryl and -alkyl 1H-isochromenes in excellent ee.
中文翻译:

通过缩酮的不对称转移氢化 有效地获得手性苯并[ c ]色烯†
建立了一种高对映选择性的合成方法,该方法通过手性亚胺二二磷酸催化相应缩酮的不对称转移加氢来获得手性α-取代的6 H-苯并[ c ]色烯。事实证明,α位置的取代基模式对不对称催化体系不敏感,具有良好耐受性的各种杂化取代基包括芳基和乙烯基,炔基和烷基部分。另外,该方法的通用性通过应用于基于1 H-异戊二烯的缩酮得到进一步证明,该酮缩醛以优异的ee提供了手性α-芳基和-烷基1 H-异色酮。
更新日期:2018-03-08
中文翻译:

通过缩酮的不对称转移氢化 有效地获得手性苯并[ c ]色烯†
建立了一种高对映选择性的合成方法,该方法通过手性亚胺二二磷酸催化相应缩酮的不对称转移加氢来获得手性α-取代的6 H-苯并[ c ]色烯。事实证明,α位置的取代基模式对不对称催化体系不敏感,具有良好耐受性的各种杂化取代基包括芳基和乙烯基,炔基和烷基部分。另外,该方法的通用性通过应用于基于1 H-异戊二烯的缩酮得到进一步证明,该酮缩醛以优异的ee提供了手性α-芳基和-烷基1 H-异色酮。











































 京公网安备 11010802027423号
京公网安备 11010802027423号