当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regioselective synthesis of unsymmetrical biheteroaryls via copper(ii)-catalyzed cascade annulation†
Chemical Communications ( IF 4.3 ) Pub Date : 2018-03-08 00:00:00 , DOI: 10.1039/c8cc00671g Sadhanendu Samanta 1, 2, 3 , Alakananda Hajra 1, 2, 3
Chemical Communications ( IF 4.3 ) Pub Date : 2018-03-08 00:00:00 , DOI: 10.1039/c8cc00671g Sadhanendu Samanta 1, 2, 3 , Alakananda Hajra 1, 2, 3
Affiliation
|
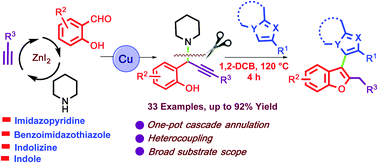
A facile and efficient copper-catalyzed cascade annulation of an imidazoheterocycle with salicylaldehyde, piperidine, and terminal alkyne was developed to afford unsymmetrical biheteroaryl derivatives. A variety of biheteroaryl structural motifs containing furans and imidazoheterocycles have been synthesized in ambient air in high yields. The experimental results suggest that the reaction proceeds through a sequential non-radical 5-exo-dig cyclization process.
中文翻译:

通过铜(ii)催化的级联环状反应 选择性合成不对称的双杂芳基†
开发了一种简便,高效的铜催化的咪唑杂环与水杨醛,哌啶和末端炔烃的级联环合反应,以制得不对称的双杂芳基衍生物。含有呋喃和咪唑杂环的各种双杂芳基结构基序已在环境空气中以高收率合成。实验结果表明,反应是通过顺序的非自由基5- exo-dig环化过程进行的。
更新日期:2018-03-08
中文翻译:

通过铜(ii)催化的级联环状反应 选择性合成不对称的双杂芳基†
开发了一种简便,高效的铜催化的咪唑杂环与水杨醛,哌啶和末端炔烃的级联环合反应,以制得不对称的双杂芳基衍生物。含有呋喃和咪唑杂环的各种双杂芳基结构基序已在环境空气中以高收率合成。实验结果表明,反应是通过顺序的非自由基5- exo-dig环化过程进行的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号