当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Size/Charge Changeable Acidity‐Responsive Micelleplex for Photodynamic‐Improved PD‐L1 Immunotherapy with Enhanced Tumor Penetration
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2018-03-08 , DOI: 10.1002/adfm.201707249 Liangliang Dai 1 , Ke Li 1 , Menghuan Li 1 , Xiaojing Zhao 1 , Zhong Luo 1 , Lu Lu 1 , Yanfeng Luo 1 , Kaiyong Cai 1, 2
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2018-03-08 , DOI: 10.1002/adfm.201707249 Liangliang Dai 1 , Ke Li 1 , Menghuan Li 1 , Xiaojing Zhao 1 , Zhong Luo 1 , Lu Lu 1 , Yanfeng Luo 1 , Kaiyong Cai 1, 2
Affiliation
|
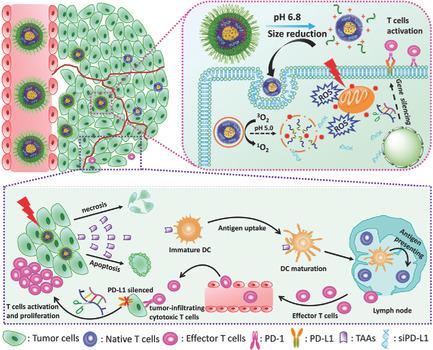
The checkpoint blockade‐based immunotherapy has recently emerged as a promising approach for tumor treatment, but its clinical implementation has been impeded by poor tumor penetration of the nanocarriers and activation of antitumor immune response. To overcome the obstacles, a tumor acidity‐responsive micellar nanocomplex co‐loaded with programmed death‐ligand 1 (PD‐L1)‐blockade siRNA and mitochondrion‐targeting photosensitizer for the synergistic integration of photodynamic therapy and immunotherapy is reported in the present study. The nanosystem is coated with long‐circulating polyethylene glycol (PEG) shells, which can be shed in response to the weakly acidic tumor microenvironment and lead to significant size reduction and increasing positive charge. These transitions facilitate penetration and uptake of nanocarriers against tumors. Subsequently, under the mild acidic endo/lysosome condition, the micellar nanocomplexes are rapidly protonated and disintegrated to release the PD‐L1‐blockade siRNA and photosensitizer through sponge effect. Results from in vitro and in vivo experiments collectively reveal that the nanosystem efficiently activates a photodynamic therapy‐induced immune response and silences immune resistance mediated by the checkpoint gene PD‐L1. In consequence, melanoma growth is inhibited and the recurrence rate is reduced via triggering systemic antitumor immune responses. This study offers an alternative strategy for the development of efficient antitumor immune therapy.
中文翻译:

大小/电荷可变的酸响应性胶束复合物,用于光动力增强的PD-L1免疫疗法,具有增强的肿瘤渗透性
基于检查点封锁的免疫疗法最近已成为一种有前途的肿瘤治疗方法,但由于纳米载体对肿瘤的不良渗透以及抗肿瘤免疫反应的激活,其临床实施受到了阻碍。为克服这些障碍,本研究报道了一种肿瘤酸反应性胶束纳米复合物,与程序性死亡配体1(PD-L1)阻断siRNA和线粒体靶向光敏剂共同负载,用于光动力疗法和免疫疗法的协同整合。纳米系统涂有长循环聚乙二醇(PEG)壳,可在弱酸性肿瘤微环境下脱落,并导致尺寸显着减小和正电荷增加。这些转变促进纳米载体对肿瘤的渗透和吸收。随后,在轻度酸性内吞/溶酶体条件下,胶束纳米复合物迅速质子化并分解,通过海绵效应释放出PD-L1阻断siRNA和光敏剂。体外和体内实验的结果共同表明,纳米系统可以有效激活光动力疗法诱导的免疫反应,并消除由检查点基因PD-L1介导的免疫抵抗。结果,通过触发全身性抗肿瘤免疫应答,抑制了黑素瘤的生长并降低了复发率。这项研究为开发有效的抗肿瘤免疫疗法提供了另一种策略。胶束纳米复合物迅速质子化并分解,通过海绵效应释放出PD-L1-blocked siRNA和光敏剂。体外和体内实验的结果共同表明,纳米系统可以有效激活光动力疗法诱导的免疫反应,并消除由检查点基因PD-L1介导的免疫抵抗。结果,通过触发全身性抗肿瘤免疫应答,抑制了黑素瘤的生长并降低了复发率。这项研究为开发有效的抗肿瘤免疫疗法提供了另一种策略。胶束纳米复合物迅速质子化并分解,通过海绵效应释放出PD-L1-blocked siRNA和光敏剂。体外和体内实验的结果共同表明,纳米系统可以有效激活光动力疗法诱导的免疫反应,并消除由检查点基因PD-L1介导的免疫抵抗。结果,通过触发全身性抗肿瘤免疫应答,抑制了黑素瘤的生长并降低了复发率。这项研究为开发有效的抗肿瘤免疫疗法提供了另一种策略。黑色素瘤的生长受到抑制,并通过触发全身性抗肿瘤免疫反应而降低了复发率。这项研究为开发有效的抗肿瘤免疫疗法提供了另一种策略。黑色素瘤的生长受到抑制,并通过触发全身性抗肿瘤免疫反应而降低了复发率。这项研究为开发有效的抗肿瘤免疫疗法提供了另一种策略。
更新日期:2018-03-08
中文翻译:

大小/电荷可变的酸响应性胶束复合物,用于光动力增强的PD-L1免疫疗法,具有增强的肿瘤渗透性
基于检查点封锁的免疫疗法最近已成为一种有前途的肿瘤治疗方法,但由于纳米载体对肿瘤的不良渗透以及抗肿瘤免疫反应的激活,其临床实施受到了阻碍。为克服这些障碍,本研究报道了一种肿瘤酸反应性胶束纳米复合物,与程序性死亡配体1(PD-L1)阻断siRNA和线粒体靶向光敏剂共同负载,用于光动力疗法和免疫疗法的协同整合。纳米系统涂有长循环聚乙二醇(PEG)壳,可在弱酸性肿瘤微环境下脱落,并导致尺寸显着减小和正电荷增加。这些转变促进纳米载体对肿瘤的渗透和吸收。随后,在轻度酸性内吞/溶酶体条件下,胶束纳米复合物迅速质子化并分解,通过海绵效应释放出PD-L1阻断siRNA和光敏剂。体外和体内实验的结果共同表明,纳米系统可以有效激活光动力疗法诱导的免疫反应,并消除由检查点基因PD-L1介导的免疫抵抗。结果,通过触发全身性抗肿瘤免疫应答,抑制了黑素瘤的生长并降低了复发率。这项研究为开发有效的抗肿瘤免疫疗法提供了另一种策略。胶束纳米复合物迅速质子化并分解,通过海绵效应释放出PD-L1-blocked siRNA和光敏剂。体外和体内实验的结果共同表明,纳米系统可以有效激活光动力疗法诱导的免疫反应,并消除由检查点基因PD-L1介导的免疫抵抗。结果,通过触发全身性抗肿瘤免疫应答,抑制了黑素瘤的生长并降低了复发率。这项研究为开发有效的抗肿瘤免疫疗法提供了另一种策略。胶束纳米复合物迅速质子化并分解,通过海绵效应释放出PD-L1-blocked siRNA和光敏剂。体外和体内实验的结果共同表明,纳米系统可以有效激活光动力疗法诱导的免疫反应,并消除由检查点基因PD-L1介导的免疫抵抗。结果,通过触发全身性抗肿瘤免疫应答,抑制了黑素瘤的生长并降低了复发率。这项研究为开发有效的抗肿瘤免疫疗法提供了另一种策略。黑色素瘤的生长受到抑制,并通过触发全身性抗肿瘤免疫反应而降低了复发率。这项研究为开发有效的抗肿瘤免疫疗法提供了另一种策略。黑色素瘤的生长受到抑制,并通过触发全身性抗肿瘤免疫反应而降低了复发率。这项研究为开发有效的抗肿瘤免疫疗法提供了另一种策略。









































 京公网安备 11010802027423号
京公网安备 11010802027423号