当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Targeting Alanines in the Hydrophobic and Cross-Linking Domains of Native Elastin with Isotopic Enrichment and Solid-State NMR Spectroscopy
Macromolecules ( IF 5.1 ) Pub Date : 2018-03-06 00:00:00 , DOI: 10.1021/acs.macromol.7b02617 Jhonsen Djajamuliadi 1 , Kosuke Ohgo 1 , Kristin K. Kumashiro 1
Macromolecules ( IF 5.1 ) Pub Date : 2018-03-06 00:00:00 , DOI: 10.1021/acs.macromol.7b02617 Jhonsen Djajamuliadi 1 , Kosuke Ohgo 1 , Kristin K. Kumashiro 1
Affiliation
|
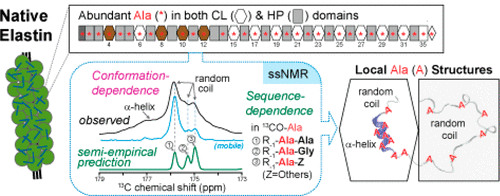
Details of elastin’s complex conformational and dynamic heterogeneity were acquired from one- and two-dimensional solid-state nuclear magnetic resonance (ssNMR) experiments on hydrated elastin that is isotopically enriched at its alanines. Elastin’s abundant alanines are useful probes of the structural and dynamical microenvironments in its cross-linking and hydrophobic domains. High isotopic enrichment of alanines in neonatal rat smooth muscle cells (NRSMC) elastin was obtained with the inhibition of alanine transaminase, combined with an excess of [U–13C]alanine in the culture media. Due to the fast, large-amplitude motions, R-TOBSY was utilized with selective homonuclear decoupling schemes to resolve 13C-Ala peaks and confirm assignments. A data-driven approach is applied, as the interpretation of chemical shifts is based on the distribution of conformation-dependent 13C-Ala chemical shifts in proteins in multiple databases. Alanine populations in elastin’s hydrophobic domains are primarily random coil, whereas those in its cross-linking regions reside in α-helices and random coils.
中文翻译:

同位素富集和固态NMR光谱法在天然弹性蛋白的疏水和交联域中靶向丙二烯
弹性蛋白的复杂构象和动态异质性的详细信息是通过对水合弹性蛋白(在其丙氨酸中同位素富集)进行的一维和二维固态核磁共振(ssNMR)实验获得的。弹性蛋白丰富的丙氨酸是其交联和疏水域中结构和动力学微环境的有用探针。通过抑制丙氨酸转氨酶和在培养基中过量的[U– 13 C]丙氨酸,可以在新生大鼠平滑肌细胞(NRSMC)弹性蛋白中获得较高的丙氨酸同位素富集度。由于快速,大振幅运动,R-TOBSY与选择性同核去耦方案一起用于解析13C-Ala峰并确认任务。应用了一种数据驱动的方法,因为化学位移的解释是基于多个数据库中蛋白质中依赖构象的13 C-Ala化学位移的分布。弹性蛋白的疏水域中的丙氨酸种群主要是无规卷曲,而在其交联区域中的丙氨酸则存在于α-螺旋和无规卷曲中。
更新日期:2018-03-06
中文翻译:

同位素富集和固态NMR光谱法在天然弹性蛋白的疏水和交联域中靶向丙二烯
弹性蛋白的复杂构象和动态异质性的详细信息是通过对水合弹性蛋白(在其丙氨酸中同位素富集)进行的一维和二维固态核磁共振(ssNMR)实验获得的。弹性蛋白丰富的丙氨酸是其交联和疏水域中结构和动力学微环境的有用探针。通过抑制丙氨酸转氨酶和在培养基中过量的[U– 13 C]丙氨酸,可以在新生大鼠平滑肌细胞(NRSMC)弹性蛋白中获得较高的丙氨酸同位素富集度。由于快速,大振幅运动,R-TOBSY与选择性同核去耦方案一起用于解析13C-Ala峰并确认任务。应用了一种数据驱动的方法,因为化学位移的解释是基于多个数据库中蛋白质中依赖构象的13 C-Ala化学位移的分布。弹性蛋白的疏水域中的丙氨酸种群主要是无规卷曲,而在其交联区域中的丙氨酸则存在于α-螺旋和无规卷曲中。











































 京公网安备 11010802027423号
京公网安备 11010802027423号