Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Glycosylated Peptoid Nanosheets as a Multivalent Scaffold for Protein Recognition
ACS Nano ( IF 15.8 ) Pub Date : 2018-03-07 00:00:00 , DOI: 10.1021/acsnano.7b08018 Alessia Battigelli 1 , Jae Hong Kim 1 , Dilani C. Dehigaspitiya 2 , Caroline Proulx 1 , Ellen J. Robertson 1 , Daniel J. Murray 1 , Behzad Rad 1 , Kent Kirshenbaum 2 , Ronald N. Zuckermann 1
ACS Nano ( IF 15.8 ) Pub Date : 2018-03-07 00:00:00 , DOI: 10.1021/acsnano.7b08018 Alessia Battigelli 1 , Jae Hong Kim 1 , Dilani C. Dehigaspitiya 2 , Caroline Proulx 1 , Ellen J. Robertson 1 , Daniel J. Murray 1 , Behzad Rad 1 , Kent Kirshenbaum 2 , Ronald N. Zuckermann 1
Affiliation
|
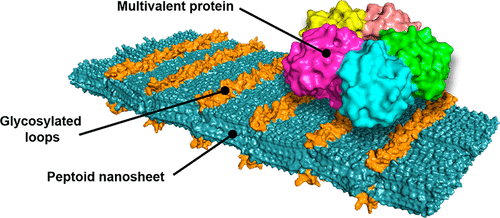
Glycoproteins adhered on the cellular membrane play a pivotal role in a wide range of cellular functions. Their importance is particularly relevant in the recognition process between infectious pathogens (such as viruses, bacteria, toxins) and their host cells. Multivalent interactions at the pathogen-cell interfaces govern binding events and can result in a strong and specific interaction. Here we report an approach to mimic the cell surface presentation of carbohydrate ligands by the multivalent display of sugars on the surface of peptoid nanosheets. The constructs provide a highly organized 2D platform for recognition of carbohydrate-binding proteins. The sugars were displayed using different linker lengths or within loops containing 2–6 hydrophilic peptoid monomers. Both the linkers and the loops contained one alkyne-bearing monomer, to which different saccharides were attached by copper-catalyzed azide–alkyne cycloaddition reactions. Peptoid nanosheets functionalized with different saccharide groups were able to selectively bind multivalent lectins, Concanavalin A and Wheat Germ Agglutinin, as observed by fluorescence microscopy and a homogeneous Förster resonance energy transfer (FRET)-based binding assay. To evaluate the potential of this system as sensor for threat agents, the ability of functionalized peptoid nanosheets to bind Shiga toxin was also studied. Peptoid nanosheets were functionalized with globotriose, the natural ligand of Shiga toxin, and the effective binding of the nanomaterial was verified by the FRET-based binding assay. In all cases, evidence for multivalent binding was observed by systematic variation of the ligand display density on the nanosheet surface. These cell surface mimetic nanomaterials may find utility in the inactivation of pathogens or as selective molecular recognition elements.
中文翻译:

糖基化的类肽纳米片作为蛋白质识别的多价支架。
粘附在细胞膜上的糖蛋白在广泛的细胞功能中起关键作用。在感染性病原体(例如病毒,细菌,毒素)与其宿主细胞之间的识别过程中,它们的重要性尤其重要。病原体-细胞界面的多价相互作用决定了结合事件,并可能导致强烈而特异性的相互作用。在这里,我们报告了一种通过类肽纳米片表面上糖的多价展示来模拟碳水化合物配体的细胞表面表现的方法。该构建体提供了高度组织化的二维平台,可识别碳水化合物结合蛋白。使用不同的接头长度或在包含2–6个亲水类肽单体的环中显示糖。连接子和环都包含一种炔烃单体,铜催化的叠氮化物-炔烃环加成反应可将不同的糖附着在其上。用不同的糖基官能化的类肽纳米片能够选择性地结合多价凝集素,伴刀豆球蛋白A和小麦胚芽凝集素,如通过荧光显微镜和基于均相福斯特共振能量转移(FRET)的结合测定所观察到的。为了评估该系统作为威胁因子传感器的潜力,还研究了功能化类肽纳米片结合志贺毒素的能力。将类肽纳米片用志贺毒素的天然配体球三糖功能化,并通过基于FRET的结合测定法验证了纳米材料的有效结合。在所有情况下,通过纳米片表面上配体显示密度的系统变化观察到多价结合的证据。这些细胞表面模拟纳米材料可用于灭活病原体或用作选择性分子识别元件。
更新日期:2018-03-07
中文翻译:

糖基化的类肽纳米片作为蛋白质识别的多价支架。
粘附在细胞膜上的糖蛋白在广泛的细胞功能中起关键作用。在感染性病原体(例如病毒,细菌,毒素)与其宿主细胞之间的识别过程中,它们的重要性尤其重要。病原体-细胞界面的多价相互作用决定了结合事件,并可能导致强烈而特异性的相互作用。在这里,我们报告了一种通过类肽纳米片表面上糖的多价展示来模拟碳水化合物配体的细胞表面表现的方法。该构建体提供了高度组织化的二维平台,可识别碳水化合物结合蛋白。使用不同的接头长度或在包含2–6个亲水类肽单体的环中显示糖。连接子和环都包含一种炔烃单体,铜催化的叠氮化物-炔烃环加成反应可将不同的糖附着在其上。用不同的糖基官能化的类肽纳米片能够选择性地结合多价凝集素,伴刀豆球蛋白A和小麦胚芽凝集素,如通过荧光显微镜和基于均相福斯特共振能量转移(FRET)的结合测定所观察到的。为了评估该系统作为威胁因子传感器的潜力,还研究了功能化类肽纳米片结合志贺毒素的能力。将类肽纳米片用志贺毒素的天然配体球三糖功能化,并通过基于FRET的结合测定法验证了纳米材料的有效结合。在所有情况下,通过纳米片表面上配体显示密度的系统变化观察到多价结合的证据。这些细胞表面模拟纳米材料可用于灭活病原体或用作选择性分子识别元件。











































 京公网安备 11010802027423号
京公网安备 11010802027423号