当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Role of zinc and magnesium ions in the modulation of phosphoryl transfer in protein tyrosine phosphatase 1B
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-03-07 , DOI: 10.1021/jacs.8b01534 Elisa Bellomo 1 , Asma Abro 2 , Christer Hogstrand 1 , Wolfgang Maret 1 , Carmen Domene 2, 3, 4
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-03-07 , DOI: 10.1021/jacs.8b01534 Elisa Bellomo 1 , Asma Abro 2 , Christer Hogstrand 1 , Wolfgang Maret 1 , Carmen Domene 2, 3, 4
Affiliation
|
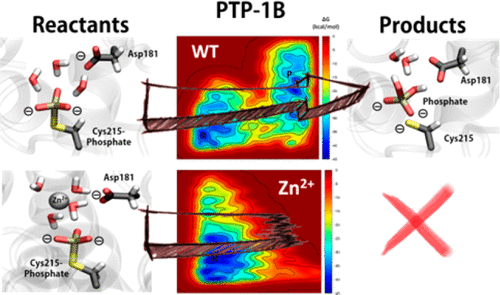
While the majority of phosphatases are metalloenzymes, the prevailing model for the reactions catalyzed by protein tyrosine phosphatases does not involve any metal ion, yet both metal cations and oxoanions affect their enzymatic activity. Mg2+ and Zn2+ activate and inhibit, respectively, protein tyrosine phosphatase 1B (PTP1B). Molecular dynamics simulations, metadynamics, and quantum chemical calculations in combination with experimental investigations demonstrate that Mg2+ and Zn2+ compete for the same binding site in the active site only in the closed conformation of the enzyme in its phosphorylated state. The two cations have different effects on the arrangements and activities of water molecules that are necessary for the hydrolysis of the phosphocysteine intermediate in the second catalytic step of the reaction. Remarkable differences between the established structural enzymology of PTP1B investigated ex vivo and the function of PTP1B in vivo become evident. Different reaction pathways are viable when the presence of metal ions and their cellular concentrations are considered. The findings suggest that the substrate delivers the inhibitory Zn2+ ion to the active site. The inhibition and activation can be ascribed to the different coordination chemistries of Zn2+ and Mg2+ ions and the orientation of the metal-coordinated water molecules. Metallochemistry adds an additional dimension to the regulation of PTP1B and presumably other members of this enzyme family.
中文翻译:

锌和镁离子在调节蛋白酪氨酸磷酸酶 1B 中磷酰基转移中的作用
虽然大多数磷酸酶是金属酶,但由蛋白质酪氨酸磷酸酶催化的反应的主流模型不涉及任何金属离子,但金属阳离子和氧阴离子都会影响它们的酶活性。Mg2+ 和 Zn2+ 分别激活和抑制蛋白酪氨酸磷酸酶 1B (PTP1B)。分子动力学模拟、元动力学和量子化学计算与实验研究相结合,表明 Mg2+ 和 Zn2+ 仅在处于磷酸化状态的酶的封闭构象中竞争活性位点中的相同结合位点。这两种阳离子对反应的第二个催化步骤中磷酸半胱氨酸中间体水解所必需的水分子的排列和活性具有不同的影响。体外研究的 PTP1B 已建立的结构酶学与体内 PTP1B 的功能之间的显着差异变得明显。当考虑金属离子的存在及其细胞浓度时,不同的反应途径是可行的。研究结果表明,底物将抑制性 Zn2+ 离子传递到活性位点。抑制和激活可归因于 Zn2+ 和 Mg2+ 离子的不同配位化学以及金属配位水分子的取向。金属化学为 PTP1B 和该酶家族的其他成员的调节增加了一个额外的维度。当考虑金属离子的存在及其细胞浓度时,不同的反应途径是可行的。研究结果表明,底物将抑制性 Zn2+ 离子传递到活性位点。抑制和激活可归因于 Zn2+ 和 Mg2+ 离子的不同配位化学以及金属配位水分子的取向。金属化学为 PTP1B 和该酶家族的其他成员的调节增加了一个额外的维度。当考虑金属离子的存在及其细胞浓度时,不同的反应途径是可行的。研究结果表明,底物将抑制性 Zn2+ 离子传递到活性位点。抑制和激活可归因于 Zn2+ 和 Mg2+ 离子的不同配位化学以及金属配位水分子的取向。金属化学为 PTP1B 和该酶家族的其他成员的调节增加了一个额外的维度。
更新日期:2018-03-07
中文翻译:

锌和镁离子在调节蛋白酪氨酸磷酸酶 1B 中磷酰基转移中的作用
虽然大多数磷酸酶是金属酶,但由蛋白质酪氨酸磷酸酶催化的反应的主流模型不涉及任何金属离子,但金属阳离子和氧阴离子都会影响它们的酶活性。Mg2+ 和 Zn2+ 分别激活和抑制蛋白酪氨酸磷酸酶 1B (PTP1B)。分子动力学模拟、元动力学和量子化学计算与实验研究相结合,表明 Mg2+ 和 Zn2+ 仅在处于磷酸化状态的酶的封闭构象中竞争活性位点中的相同结合位点。这两种阳离子对反应的第二个催化步骤中磷酸半胱氨酸中间体水解所必需的水分子的排列和活性具有不同的影响。体外研究的 PTP1B 已建立的结构酶学与体内 PTP1B 的功能之间的显着差异变得明显。当考虑金属离子的存在及其细胞浓度时,不同的反应途径是可行的。研究结果表明,底物将抑制性 Zn2+ 离子传递到活性位点。抑制和激活可归因于 Zn2+ 和 Mg2+ 离子的不同配位化学以及金属配位水分子的取向。金属化学为 PTP1B 和该酶家族的其他成员的调节增加了一个额外的维度。当考虑金属离子的存在及其细胞浓度时,不同的反应途径是可行的。研究结果表明,底物将抑制性 Zn2+ 离子传递到活性位点。抑制和激活可归因于 Zn2+ 和 Mg2+ 离子的不同配位化学以及金属配位水分子的取向。金属化学为 PTP1B 和该酶家族的其他成员的调节增加了一个额外的维度。当考虑金属离子的存在及其细胞浓度时,不同的反应途径是可行的。研究结果表明,底物将抑制性 Zn2+ 离子传递到活性位点。抑制和激活可归因于 Zn2+ 和 Mg2+ 离子的不同配位化学以及金属配位水分子的取向。金属化学为 PTP1B 和该酶家族的其他成员的调节增加了一个额外的维度。











































 京公网安备 11010802027423号
京公网安备 11010802027423号