当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reversing the Tradeoff between Rate and Overpotential in Molecular Electrocatalysts for H2 Production
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-03-07 00:00:00 , DOI: 10.1021/acscatal.7b04379 Christina M. Klug 1 , Allan Jay P. Cardenas 1 , R. Morris Bullock 1 , Molly O’Hagan 1 , Eric S. Wiedner 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-03-07 00:00:00 , DOI: 10.1021/acscatal.7b04379 Christina M. Klug 1 , Allan Jay P. Cardenas 1 , R. Morris Bullock 1 , Molly O’Hagan 1 , Eric S. Wiedner 1
Affiliation
|
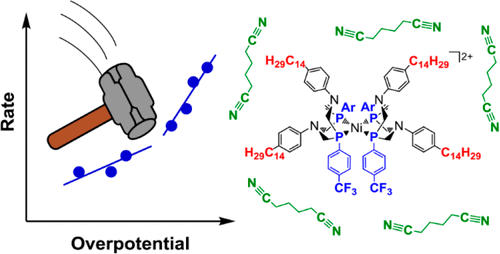
A longstanding challenge in molecular electrocatalysis is to design catalysts that break away from the tradeoff between rate and overpotential arising from electronic scaling relationships. Here we report an inversion of the rate–overpotential correlation through system-level design of [Ni(PR2NR′2)2]2+ electrocatalysts for the production of H2. The overpotential is lowered by an electron-withdrawing ligand, while the turnover frequency is increased by controlling the catalyst structural dynamics, using both ligand design and solvent viscosity. The cumulative effect of controlling each of these system components is an electrocatalyst with a turnover frequency of 70000 s–1 and an overpotential of 230 mV, corresponding to a 100-fold rate enhancement and a 170 mV reduction in overpotential in comparison to the parent nickel catalyst. Molecular Tafel plot analysis reveals that the new catalysts reported here are substantially more efficient than other leading molecular electrocatalysts for the production of H2.
中文翻译:

逆转用于生产H 2的分子电催化剂中速率与超电势之间的权衡
分子电催化中的长期挑战是设计出能够摆脱速率和电子比例关系引起的过电位之间的折衷的催化剂。在这里,我们通过[镍(P的系统级设计报告速率过电位相关的反转- [R 2 Ñ - [R ' 2)2 ] 2+电催化剂用于生产h的2。吸电子配体可降低过电势,而配体设计和溶剂粘度均可通过控制催化剂的结构动力学来提高转换频率。控制这些系统组件中每一个的累积效果是一种电子催化剂,其转换频率为70000 s–1和230 mV的超电势,与母体镍催化剂相比,相当于速率增加了100倍,超电势降低了170 mV。分子塔菲尔曲线分析表明,此处报道的新催化剂比其他领先的分子电催化剂在生产H 2方面要高效得多。
更新日期:2018-03-07
中文翻译:

逆转用于生产H 2的分子电催化剂中速率与超电势之间的权衡
分子电催化中的长期挑战是设计出能够摆脱速率和电子比例关系引起的过电位之间的折衷的催化剂。在这里,我们通过[镍(P的系统级设计报告速率过电位相关的反转- [R 2 Ñ - [R ' 2)2 ] 2+电催化剂用于生产h的2。吸电子配体可降低过电势,而配体设计和溶剂粘度均可通过控制催化剂的结构动力学来提高转换频率。控制这些系统组件中每一个的累积效果是一种电子催化剂,其转换频率为70000 s–1和230 mV的超电势,与母体镍催化剂相比,相当于速率增加了100倍,超电势降低了170 mV。分子塔菲尔曲线分析表明,此处报道的新催化剂比其他领先的分子电催化剂在生产H 2方面要高效得多。











































 京公网安备 11010802027423号
京公网安备 11010802027423号