当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Influence of Polymer and Drug Loading on the Release Profile and Membrane Transport of Telaprevir
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-03-07 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00104 Laura I. Mosquera-Giraldo 1 , Na Li 1 , Venecia R. Wilson 1 , Brittany L.B. Nichols 2, 3 , Kevin J. Edgar 3 , Lynne S. Taylor 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-03-07 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00104 Laura I. Mosquera-Giraldo 1 , Na Li 1 , Venecia R. Wilson 1 , Brittany L.B. Nichols 2, 3 , Kevin J. Edgar 3 , Lynne S. Taylor 1
Affiliation
|
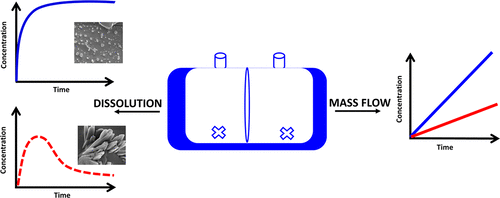
During the dissolution of amorphous solid dispersions (ASDs), various phase transformations can occur, which will ultimately impact the degree of supersaturation. This study employed dissolution and diffusion measurements to compare the performance of various ASD formulations based on the maximum amount of free drug in the solution that was able to permeate through a cellulose-based membrane. Telaprevir (TPV) was used as the model drug compound, and ASDs were prepared with different drug loadings and with four different polymers. Four possible scenarios that can influence TPV mass flow rates upon ASD dissolution were described and supported with experimental data: (1) a system dissolves readily and completely undergoes phase separation via glass–liquid phase separation (GLPS), forming drug-rich aggregates, and reaches the maximum anticipated mass flow rate; (2) where the maximum mass flow rate decreases due to substantial mixing of the polymer into the drug-rich phase, and/or due to the formation of soluble polymer–drug complexes; (3) a system does not undergo GLPS due to slow drug release and/or matrix crystallization; and (4) a system does not undergo GLPS due to rapid crystallization from the supersaturated solution generated during dissolution. The results described herein support the importance of the combined use of the dissolution–diffusion measurements to determine the maximum level of supersaturation achievable for diverse drug formulations.
中文翻译:

聚合物和药物负载量对Telaprevir释放曲线和膜转运的影响
在非晶态固体分散体(ASD)的溶解过程中,会发生各种相变,这最终会影响过饱和度。这项研究采用溶出度和扩散度测量结果,根据溶液中能够透过纤维素膜的最大游离药物量,比较了各种ASD制剂的性能。将特拉普韦(TPV)用作模型药物化合物,并制备了具有不同载药量和四种不同聚合物的ASD。描述了四种可能影响ASD溶解后TPV质量流速的情况,并得到了实验数据的支持:(1)系统易于溶解,并通过玻璃-液相分离(GLPS)完全经历了相分离,形成了富含药物的聚集体,并达到最大预期质量流量;(2)最大质量流速下降是由于聚合物大量混合到药物富集相中,和/或由于形成了可溶性聚合物-药物复合物;(3)由于药物释放缓慢和/或基质结晶,系统未经历GLPS;(4)由于在溶解过程中产生的过饱和溶液中快速结晶,因此系统未经历GLPS。本文所述结果支持溶出-扩散测量结合使用以确定各种药物制剂可达到的最大过饱和度最高水平的重要性。(3)由于药物释放缓慢和/或基质结晶,系统未经历GLPS;(4)由于在溶解过程中产生的过饱和溶液中快速结晶,因此系统未经历GLPS。本文所述结果支持溶出-扩散测量结合使用以确定各种药物制剂可达到的最大过饱和度水平的重要性。(3)由于药物释放缓慢和/或基质结晶,系统未经历GLPS;(4)由于在溶解过程中产生的过饱和溶液中快速结晶,因此系统未经历GLPS。本文所述结果支持溶出-扩散测量结合使用以确定各种药物制剂可达到的最大过饱和度水平的重要性。
更新日期:2018-03-07
中文翻译:

聚合物和药物负载量对Telaprevir释放曲线和膜转运的影响
在非晶态固体分散体(ASD)的溶解过程中,会发生各种相变,这最终会影响过饱和度。这项研究采用溶出度和扩散度测量结果,根据溶液中能够透过纤维素膜的最大游离药物量,比较了各种ASD制剂的性能。将特拉普韦(TPV)用作模型药物化合物,并制备了具有不同载药量和四种不同聚合物的ASD。描述了四种可能影响ASD溶解后TPV质量流速的情况,并得到了实验数据的支持:(1)系统易于溶解,并通过玻璃-液相分离(GLPS)完全经历了相分离,形成了富含药物的聚集体,并达到最大预期质量流量;(2)最大质量流速下降是由于聚合物大量混合到药物富集相中,和/或由于形成了可溶性聚合物-药物复合物;(3)由于药物释放缓慢和/或基质结晶,系统未经历GLPS;(4)由于在溶解过程中产生的过饱和溶液中快速结晶,因此系统未经历GLPS。本文所述结果支持溶出-扩散测量结合使用以确定各种药物制剂可达到的最大过饱和度最高水平的重要性。(3)由于药物释放缓慢和/或基质结晶,系统未经历GLPS;(4)由于在溶解过程中产生的过饱和溶液中快速结晶,因此系统未经历GLPS。本文所述结果支持溶出-扩散测量结合使用以确定各种药物制剂可达到的最大过饱和度水平的重要性。(3)由于药物释放缓慢和/或基质结晶,系统未经历GLPS;(4)由于在溶解过程中产生的过饱和溶液中快速结晶,因此系统未经历GLPS。本文所述结果支持溶出-扩散测量结合使用以确定各种药物制剂可达到的最大过饱和度水平的重要性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号