当前位置:
X-MOL 学术
›
ChemSusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Aerobic Oxidation of 5‐Hydroxymethylfurfural to 2,5‐Diformylfuran or 2‐Formyl‐5‐furancarboxylic Acid in Water by using MgO⋅CeO2 Mixed Oxides as Catalysts
ChemSusChem ( IF 7.5 ) Pub Date : 2018-04-06 , DOI: 10.1002/cssc.201800334 Maria Ventura 1 , Francesco Lobefaro 2 , Elvira de Giglio 3 , Monica Distaso 4 , Francesco Nocito 3 , Angela Dibenedetto 1, 3
ChemSusChem ( IF 7.5 ) Pub Date : 2018-04-06 , DOI: 10.1002/cssc.201800334 Maria Ventura 1 , Francesco Lobefaro 2 , Elvira de Giglio 3 , Monica Distaso 4 , Francesco Nocito 3 , Angela Dibenedetto 1, 3
Affiliation
|
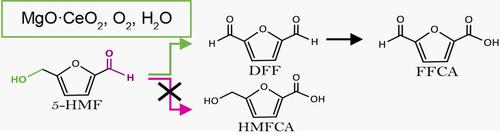
Mixed oxides based on MgO⋅CeO2 were used as efficient catalysts in the aerobic oxidation of 5‐hydroxymethylfurfural (5‐HMF) to afford, with very high selectivity, either 2,5‐diformylfuran (DFF, 99 %) or 2‐formyl‐5‐furancarboxylic acid (FFCA, 90 %), depending on the reaction conditions. 5‐Hydroxymethyl‐2‐furancarboxylic acid (HMFCA, 57–90 %) was formed only at low concentration of 5‐HMF (<0.03 m) or in presence of external bases. The conversion of 5‐HMF ranged from a few percent to 99 %, according to the reaction conditions. The oxidation was performed in water, with O2 as oxidant, without any additives. The surface characterization of the catalysts gave important information about their acid–base properties, which drive the selectivity of the reaction towards DFF. FFCA was formed from DFF at longer reaction times. Catalysts were studied by XPS and XRD before and after catalytic runs to identify the reason why they undergo reversible deactivation. XRD showed that MgO is hydrated to Mg(OH)2, which, even if not leached out, changes the basic properties of the catalyst that becomes less active after some time. Calcination of the recovered catalyst allows recovery of its initial activity. The catalyst is thus recoverable (>99 %) and reusable. The use of mixed oxides allows tuning of the basicity of the catalysts, avoiding the need for external bases for efficient and selective conversion of 5‐HMF and waste formation, resulting in an environmentally friendly, sustainable process.
中文翻译:

以MgO·CeO2混合氧化物为催化剂的水中5-羟甲基糠醛选择性好氧氧化为2,5-二甲酰基呋喃或2-甲酰基-5-呋喃羧酸
基于MgO · CeO 2的混合氧化物在5-羟甲基糠醛(5-HMF)的好氧氧化中用作有效的催化剂,以非常高的选择性提供2,5-二甲酰呋喃(DFF,99%)或2-甲酰-5-呋喃甲酸(FFCA,90%),取决于反应条件。5-羟甲基-2-呋喃甲酸(HMFCA,57-90%)仅在低浓度的5-HMF(<0.03 m)或存在外部碱的情况下形成。根据反应条件,5-HMF的转化率范围从百分之几到99%。在水中用O 2进行氧化作为氧化剂,无任何添加剂。催化剂的表面表征提供了有关其酸碱性质的重要信息,这些性质推动了反应对DFF的选择性。FFCA是由DFF在更长的反应时间形成的。在催化运行之前和之后,通过XPS和XRD对催化剂进行了研究,以确定它们经历可逆失活的原因。XRD显示MgO被水合为Mg(OH)2,即使没有浸出,其也会改变催化剂的基本性质,使其在一段时间后变得不太活跃。回收的催化剂的煅烧允许其初始活性的恢复。因此,催化剂是可回收的(> 99%)并且可重复使用。使用混合氧化物可以调节催化剂的碱度,避免需要外部碱来有效和选择性地转化5-HMF和形成废物,从而实现了环境友好,可持续的工艺。
更新日期:2018-04-06
中文翻译:

以MgO·CeO2混合氧化物为催化剂的水中5-羟甲基糠醛选择性好氧氧化为2,5-二甲酰基呋喃或2-甲酰基-5-呋喃羧酸
基于MgO · CeO 2的混合氧化物在5-羟甲基糠醛(5-HMF)的好氧氧化中用作有效的催化剂,以非常高的选择性提供2,5-二甲酰呋喃(DFF,99%)或2-甲酰-5-呋喃甲酸(FFCA,90%),取决于反应条件。5-羟甲基-2-呋喃甲酸(HMFCA,57-90%)仅在低浓度的5-HMF(<0.03 m)或存在外部碱的情况下形成。根据反应条件,5-HMF的转化率范围从百分之几到99%。在水中用O 2进行氧化作为氧化剂,无任何添加剂。催化剂的表面表征提供了有关其酸碱性质的重要信息,这些性质推动了反应对DFF的选择性。FFCA是由DFF在更长的反应时间形成的。在催化运行之前和之后,通过XPS和XRD对催化剂进行了研究,以确定它们经历可逆失活的原因。XRD显示MgO被水合为Mg(OH)2,即使没有浸出,其也会改变催化剂的基本性质,使其在一段时间后变得不太活跃。回收的催化剂的煅烧允许其初始活性的恢复。因此,催化剂是可回收的(> 99%)并且可重复使用。使用混合氧化物可以调节催化剂的碱度,避免需要外部碱来有效和选择性地转化5-HMF和形成废物,从而实现了环境友好,可持续的工艺。











































 京公网安备 11010802027423号
京公网安备 11010802027423号