当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalyst-controlled synthesis of 4-amino-isoquinolin-1(2H)-one and oxazole derivatives†
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-03-08 00:00:00 , DOI: 10.1039/c8qo00125a Ben Niu 1, 2, 3, 4, 5 , Ruixing Liu 6, 7, 8, 9, 10 , Yin Wei 6, 7, 8, 9, 10 , Min Shi 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-03-08 00:00:00 , DOI: 10.1039/c8qo00125a Ben Niu 1, 2, 3, 4, 5 , Ruixing Liu 6, 7, 8, 9, 10 , Yin Wei 6, 7, 8, 9, 10 , Min Shi 1, 2, 3, 4, 5
Affiliation
|
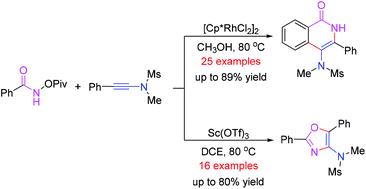
A facile synthetic method to access 4-amino-isoquinolin-1(2H)-one and oxazole derivatives was disclosed in this paper. The reaction of N-(pivaloyloxy)-amides with ynamides produced 4-amino-isoquinolin-1(2H)-ones in good yields in the presence of Cp*Rh(III) catalyst through a C–H bond functionalization. Using Sc(OTf)3 as the catalyst, oxazole derivatives were obtained in good yields from the same reaction. This protocol features good functional group tolerance and excellent regioselectivity.
中文翻译:

催化剂控制的4-氨基-异喹啉-1(2 H)-one和恶唑衍生物的合成†
本文公开了一种简便的合成方法,用于获得4-氨基-异喹啉-1(2 H)-one和恶唑衍生物。在Cp * Rh(III)催化剂存在下,通过C–H键官能化,N-(新戊酰氧基)-酰胺与乙酰胺的反应以高收率产生了4-氨基-异喹啉-1(2 H)- 。使用Sc(OTf)3作为催化剂,可以从同一反应中以高收率获得恶唑衍生物。该方案具有良好的功能基团耐受性和出色的区域选择性。
更新日期:2018-03-08
中文翻译:

催化剂控制的4-氨基-异喹啉-1(2 H)-one和恶唑衍生物的合成†
本文公开了一种简便的合成方法,用于获得4-氨基-异喹啉-1(2 H)-one和恶唑衍生物。在Cp * Rh(III)催化剂存在下,通过C–H键官能化,N-(新戊酰氧基)-酰胺与乙酰胺的反应以高收率产生了4-氨基-异喹啉-1(2 H)- 。使用Sc(OTf)3作为催化剂,可以从同一反应中以高收率获得恶唑衍生物。该方案具有良好的功能基团耐受性和出色的区域选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号