当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effect of Ligand Modification on the Mechanism of Electrocatalytic Hydrogen Production by Ni(pyridinethiolate)3– Derivatives
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-02-28 00:00:00 , DOI: 10.1021/acs.jpca.7b11912 C. N. Virca 1 , J. R. Lohmolder 1 , J. B. Tsang 1 , M. M. Davis 1 , T. M. McCormick 1
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-02-28 00:00:00 , DOI: 10.1021/acs.jpca.7b11912 C. N. Virca 1 , J. R. Lohmolder 1 , J. B. Tsang 1 , M. M. Davis 1 , T. M. McCormick 1
Affiliation
|
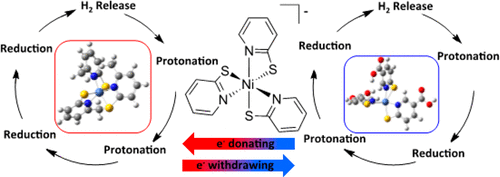
The effects of ligand modification on the catalytic mechanism of hydrogen production by Ni(PyS)3– derivatives, made with electron-withdrawing and -donating substitutions to the pyridinethiolate (PyS)− ligands, are studied experimentally and computationally using density functional theory. Thermodynamic data, spin density maps, and frontier molecular orbital diagrams were generated for reaction intermediates. Comparison of computed values for E0 and pKa with experimental values supports the proposed mechanisms. The rate of electrochemical hydrogen production is correlated with the effect of ligand modification. Notably, the presence of an electron-donating substituent favors an alternative mechanism for hydrogen production. Computationally it was determined that the electron-donating substituent causes deviation from the original chemical–electrochemical–chemical–electrochemical (CECE) mechanism of Ni(PyS)3– to a CCEE mechanism, while the CECE mechanism is maintained for all catalysts substituted with electron-withdrawing groups.
中文翻译:

配位基修饰的对电制氢用Ni(基吡啶)机制3 -衍生物
配体修饰对用Ni(PYS)制氢的催化机制的影响3 -衍生物,具有吸电子和-donating取代的基吡啶(PYS)制成-配体,进行了研究和实验计算使用密度泛函理论。生成了反应中间体的热力学数据,自旋密度图和前沿分子轨道图。E 0和p K a的计算值比较实验值支持所提出的机制。电化学氢产生的速率与配体修饰的作用相关。值得注意的是,给电子取代基的存在有利于产生氢的另一种机理。计算它被确定给电子取代基会导致偏差选自Ni(PYS)的原始化学电化学化学电化学(CECE)机构3 -至CCEE机构,而CECE机构维持用于与电子取代所有催化剂-撤军团体。
更新日期:2018-02-28
中文翻译:

配位基修饰的对电制氢用Ni(基吡啶)机制3 -衍生物
配体修饰对用Ni(PYS)制氢的催化机制的影响3 -衍生物,具有吸电子和-donating取代的基吡啶(PYS)制成-配体,进行了研究和实验计算使用密度泛函理论。生成了反应中间体的热力学数据,自旋密度图和前沿分子轨道图。E 0和p K a的计算值比较实验值支持所提出的机制。电化学氢产生的速率与配体修饰的作用相关。值得注意的是,给电子取代基的存在有利于产生氢的另一种机理。计算它被确定给电子取代基会导致偏差选自Ni(PYS)的原始化学电化学化学电化学(CECE)机构3 -至CCEE机构,而CECE机构维持用于与电子取代所有催化剂-撤军团体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号