当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rate Constants for the Reactions of OH Radical with the (E)/(Z) Isomers of CF3CF═CHCl and CHF2CF═CHCl
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-03-07 00:00:00 , DOI: 10.1021/acs.jpca.7b11923 Kazuaki Tokuhashi 1 , Tadafumi Uchimaru 1 , Kenji Takizawa 1 , Shigeo Kondo 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-03-07 00:00:00 , DOI: 10.1021/acs.jpca.7b11923 Kazuaki Tokuhashi 1 , Tadafumi Uchimaru 1 , Kenji Takizawa 1 , Shigeo Kondo 1
Affiliation
|
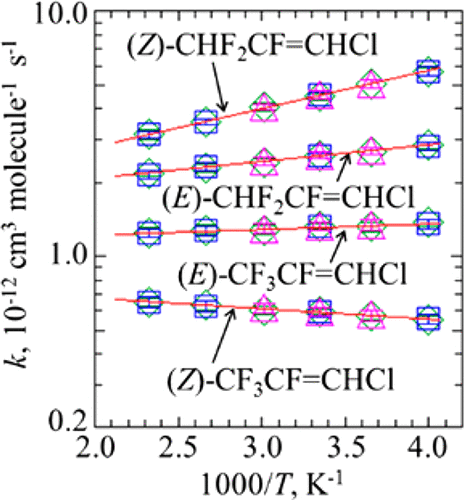
The rate constants for the reactions of OH radical with (E)- and (Z)-isomers of CF3CF═CHCl and CHF2CF═CHCl have been measured over the temperature range of 250–430 K. Kinetic measurements have been performed using flash and laser photolysis methods combined with laser-induced fluorescence. Arrhenius rate constants have been determined as k((E)-CF3CF═CHCl) = (1.09 ± 0.03) × 10–12 · exp[(50 ± 10)K/T], k((Z)-CF3CF═CHCl) = (8.02 ± 0.19) × 10–13 · exp[−(100 ± 10)K/T], k((E)–CHF2CF═CHCl) = (1.50 ± 0.03) × 10–12 · exp[(160 ± 10)K/T], and k((Z)–CHF2CF═CHCl) = (1.36 ± 0.03) × 10–12 · exp[(360 ± 10)K/T] cm3 molecule–1 s–1. Infrared absorption spectra have also been measured at room temperature. The atmospheric lifetimes of (E)-CF3CF═CHCl, (Z)-CF3CF═CHCl, (E)-CHF2CF═CHCl, and (Z)-CHF2CF═CHCl have been estimated as 8.9, 20, 4.6, and 2.6 days, respectively, and their global warming potentials and ozone depletion potentials were determined as 0.23, 0.88, 0.060, and 0.016 and 0.00010, 0.00023, 0.000057, and 0.000030, respectively. Additionally, the rate constants for OH radical addition and IR spectra of these compounds were determined computationally. Consistent with experiment, our calculations indicate that the reactivity toward OH radical addition is reduced as (Z)-CHF2CF═CHCl > (E)-CHF2CF═CHCl > (E)-CF3CF═CHCl > (Z)-CF3CF═CHCl, where the (E)/(Z) reactivity is reversed for CF3CF═CHCl and CHF2CF═CHCl. The calculations reproduced the observed temperature dependencies of the rate constants for the OH radical reactions, which is slightly positive for (Z)-CF3CF═CHCl but negative for the other compounds.
中文翻译:

速率常数的OH自由基与(反应ë)/(Ž)异构体的CF的3 CF═CHCl和CHF 2 CF═CHCl
为OH的基团与(反应的速率常数ë) -和(Ž)CF -异构体的3 CF═CHCl和CHF 2 CF═CHCl已在温度范围内的250-430 K.动力学测量来测量已经被执行使用闪光灯和激光光解方法结合激光诱导的荧光。阿列纽斯速率常数已被确定为ķ((ë)-CF 3 CF═CHCl)=(1.09±0.03)×10 -12 ·EXP [(50±10)K / Ť ] ķ((ż)-CF 3 CF═CHCl)=(8.02±0.19)×10 –13 ·exp [-(100±10)K / T ],k((ë)-CHF 2 CF═CHCl)=(1.50±0.03)×10 -12 ·EXP [(160±10)K / Ť ],和ķ((ż)-CHF 2 CF═CHCl)=(1.36 ±0.03)×10 –12 ·exp [(360±10)K / T ] cm 3分子–1 s –1。还已经在室温下测量了红外吸收光谱。(的大气寿命ë)-CF 3 CF═CHCl,(Ž)-CF 3 CF═CHCl,(ë)-CHF 2 CF═CHCl,和(Ž)-CHF 2CF═CHCl的估计天数分别为8.9天,20天,4.6天和2.6天,其全球变暖潜势和臭氧消耗潜能分别确定为0.23、0.88、0.060和0.016和0.00010、0.00023、0.000057和0.000030 。另外,通过计算确定了这些化合物的OH自由基加成的速率常数和IR光谱。与实验一致,我们的计算表明,朝向OH基加反应性降低为(ż)-CHF 2 CF═CHCl>(ë)-CHF 2 CF═CHCl>(ë)-CF 3 CF═CHCl>(Ž) -CF 3 CF═CHCl,其中(ë)/(ž)反应性反转CF 3 CF═CHCl和CHF 2 CF═CHCl。计算再现的速率常数的观测温度的依赖关系为OH自由基反应,这对于(略正Ž)-CF 3 CF═CHCl但阴性为其它化合物。
更新日期:2018-03-07
中文翻译:

速率常数的OH自由基与(反应ë)/(Ž)异构体的CF的3 CF═CHCl和CHF 2 CF═CHCl
为OH的基团与(反应的速率常数ë) -和(Ž)CF -异构体的3 CF═CHCl和CHF 2 CF═CHCl已在温度范围内的250-430 K.动力学测量来测量已经被执行使用闪光灯和激光光解方法结合激光诱导的荧光。阿列纽斯速率常数已被确定为ķ((ë)-CF 3 CF═CHCl)=(1.09±0.03)×10 -12 ·EXP [(50±10)K / Ť ] ķ((ż)-CF 3 CF═CHCl)=(8.02±0.19)×10 –13 ·exp [-(100±10)K / T ],k((ë)-CHF 2 CF═CHCl)=(1.50±0.03)×10 -12 ·EXP [(160±10)K / Ť ],和ķ((ż)-CHF 2 CF═CHCl)=(1.36 ±0.03)×10 –12 ·exp [(360±10)K / T ] cm 3分子–1 s –1。还已经在室温下测量了红外吸收光谱。(的大气寿命ë)-CF 3 CF═CHCl,(Ž)-CF 3 CF═CHCl,(ë)-CHF 2 CF═CHCl,和(Ž)-CHF 2CF═CHCl的估计天数分别为8.9天,20天,4.6天和2.6天,其全球变暖潜势和臭氧消耗潜能分别确定为0.23、0.88、0.060和0.016和0.00010、0.00023、0.000057和0.000030 。另外,通过计算确定了这些化合物的OH自由基加成的速率常数和IR光谱。与实验一致,我们的计算表明,朝向OH基加反应性降低为(ż)-CHF 2 CF═CHCl>(ë)-CHF 2 CF═CHCl>(ë)-CF 3 CF═CHCl>(Ž) -CF 3 CF═CHCl,其中(ë)/(ž)反应性反转CF 3 CF═CHCl和CHF 2 CF═CHCl。计算再现的速率常数的观测温度的依赖关系为OH自由基反应,这对于(略正Ž)-CF 3 CF═CHCl但阴性为其它化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号