Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nanopore Opening at Flat and Nanotip Conical Electrodes during Vesicle Impact Electrochemical Cytometry
ACS Nano ( IF 15.8 ) Pub Date : 2018-03-07 00:00:00 , DOI: 10.1021/acsnano.8b00781 Xianchan Li 1 , Lin Ren 2 , Johan Dunevall 2 , Daixin Ye 1 , Henry S. White 3 , Martin A. Edwards 3 , Andrew G. Ewing 1, 2
ACS Nano ( IF 15.8 ) Pub Date : 2018-03-07 00:00:00 , DOI: 10.1021/acsnano.8b00781 Xianchan Li 1 , Lin Ren 2 , Johan Dunevall 2 , Daixin Ye 1 , Henry S. White 3 , Martin A. Edwards 3 , Andrew G. Ewing 1, 2
Affiliation
|
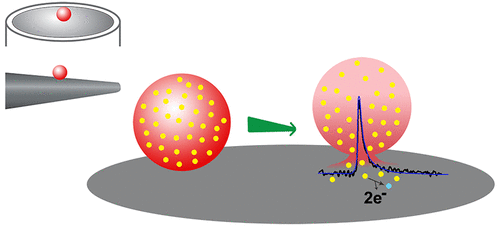
The oxidation of catecholamine at a microelectrode, following its release from individual vesicles, allows interrogation of the content of single nanometer vesicles with vesicle impact electrochemical cytometry (VIEC). Previous to this development, there were no methods available to quantify the chemical load of single vesicles. However, accurate quantification of the content is hampered by uncertainty in the proportion of substituent molecules reaching the electrode surface (collection efficiency). In this work, we use quantitative modeling to calculate this collection efficiency. For all vesicles except those at the very edge of the electrode, modeling shows that ∼100% oxidation efficiency is achieved when employing a 33 μm diameter disk microelectrode for VIEC, independent of the location of the vesicle release pore. We use this to experimentally determine a precise distribution of catecholamine in individual vesicles extracted from PC12 cells. In contrast, we calculate that when a nanotip conical electrode (∼4 μm length, ∼1.5 μm diameter at the base) is employed, as in intracellular VIEC (IVIEC), the current–time response depends strongly on the position of the catecholamine-releasing pore in the vesicle membrane. When vesicle release occurs with the pore opening occurring far from the electrode, lower currents and partial oxidation (∼75%) of the catecholamine are predicted, as compared to higher currents and ∼100% oxidation, when the pore is close to/at the electrode surface. As close agreement is observed between the experimentally measured vesicular content in intracellular and extracted vesicles from the same cell line using nanotip and disk electrodes, respectively, we conclude that pores open at the electrode surface. Not only does this suggest that electroporation of the vesicle membrane is the primary driving force for catecholamine release from vesicles at polarized electrodes, but it also indicates that IVIEC with nanotip electrodes can directly assess vesicular content without correction.
中文翻译:

囊泡冲击电化学细胞计数过程中在扁平和纳米尖端锥形电极上的纳米孔开口。
儿茶酚胺从单个囊泡中释放后,在微电极上发生氧化,可通过囊泡冲击电化学细胞计数法(VIEC)询问单个纳米囊泡的含量。在此发展之前,还没有可用的方法来量化单个囊泡的化学负荷。但是,由于到达电极表面的取代基分子的比例(收集效率)的不确定性,妨碍了含量的准确定量。在这项工作中,我们使用定量建模来计算收集效率。对于所有囊泡,除了电极最边缘的囊泡,建模显示,当将33μm直径的圆盘微电极用于VIEC时,氧化效率约为100%,而与囊泡释放孔的位置无关。我们用它来实验确定儿茶酚胺在从PC12细胞提取的单个囊泡中的精确分布。相反,我们计算出,当采用纳米尖端的锥形电极(长度约为4μm,底部直径约为1.5μm)时,如细胞内VIEC(IVIEC)一样,电流-时间响应在很大程度上取决于儿茶酚胺的位置。释放囊泡膜中的孔。当在远离电极的开孔处发生囊泡释放时,与之相比,当孔靠近/处于开孔状态时,与较高的电流和〜100%的氧化相比,儿茶酚胺的较低的电流和部分氧化(〜75%)被预测。电极表面。由于使用纳米尖端和盘状电极在同一细胞系中实验测量的囊泡含量和从同一细胞系提取的囊泡中的实验测量值之间存在密切的一致性,分别得出结论,孔在电极表面开口。这不仅表明囊泡膜的电穿孔是极化电极上儿茶酚胺从囊泡释放的主要驱动力,而且还表明具有纳米尖端电极的IVIEC无需校正即可直接评估囊泡含量。
更新日期:2018-03-07
中文翻译:

囊泡冲击电化学细胞计数过程中在扁平和纳米尖端锥形电极上的纳米孔开口。
儿茶酚胺从单个囊泡中释放后,在微电极上发生氧化,可通过囊泡冲击电化学细胞计数法(VIEC)询问单个纳米囊泡的含量。在此发展之前,还没有可用的方法来量化单个囊泡的化学负荷。但是,由于到达电极表面的取代基分子的比例(收集效率)的不确定性,妨碍了含量的准确定量。在这项工作中,我们使用定量建模来计算收集效率。对于所有囊泡,除了电极最边缘的囊泡,建模显示,当将33μm直径的圆盘微电极用于VIEC时,氧化效率约为100%,而与囊泡释放孔的位置无关。我们用它来实验确定儿茶酚胺在从PC12细胞提取的单个囊泡中的精确分布。相反,我们计算出,当采用纳米尖端的锥形电极(长度约为4μm,底部直径约为1.5μm)时,如细胞内VIEC(IVIEC)一样,电流-时间响应在很大程度上取决于儿茶酚胺的位置。释放囊泡膜中的孔。当在远离电极的开孔处发生囊泡释放时,与之相比,当孔靠近/处于开孔状态时,与较高的电流和〜100%的氧化相比,儿茶酚胺的较低的电流和部分氧化(〜75%)被预测。电极表面。由于使用纳米尖端和盘状电极在同一细胞系中实验测量的囊泡含量和从同一细胞系提取的囊泡中的实验测量值之间存在密切的一致性,分别得出结论,孔在电极表面开口。这不仅表明囊泡膜的电穿孔是极化电极上儿茶酚胺从囊泡释放的主要驱动力,而且还表明具有纳米尖端电极的IVIEC无需校正即可直接评估囊泡含量。











































 京公网安备 11010802027423号
京公网安备 11010802027423号