当前位置:
X-MOL 学术
›
ChemElectroChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Radical Diazidation of Alkenes: Cu/Fe/Mn Catalysis and Electrochemical Support
ChemElectroChem ( IF 3.5 ) Pub Date : 2018-03-07 , DOI: 10.1002/celc.201800160 Nisar Ahmed 1 , Saira Khatoon 1 , Bahareh Shirinfar 2
ChemElectroChem ( IF 3.5 ) Pub Date : 2018-03-07 , DOI: 10.1002/celc.201800160 Nisar Ahmed 1 , Saira Khatoon 1 , Bahareh Shirinfar 2
Affiliation
|
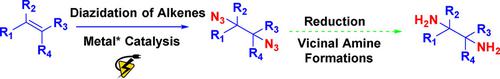
The synthetic routes for C‐N3 bond formation through a radical mechanism with the use of transition metals provide efficient diazide products, which can be further applied in many transformations to synthesize various valuable nitrogen‐containing compounds of pharmaceutical interest. The reported methods evidence stoichiometric heavy metals and toxic reagents (as N3‐source) and generally exhibit a limited substrate scope. However, the electrochemical diazidations are found to be environmentally friendly and very simple, while also providing a highly efficient way to synthesize 1,2‐diazide derivatives. Moreover, they provide a smart alternative for easy access to 1,2‐diamines, which can be used for future modern syntheses.
中文翻译:

烯烃的自由基重氮化:Cu / Fe / Mn催化和电化学支持
通过使用过渡金属通过自由基机理形成C-N 3键的合成途径可提供高效的叠氮化物产物,该产物可进一步用于许多转化中,以合成各种有价值的药用含氮化合物。报告的方法证明了化学计量的重金属和有毒试剂(以N 3为来源),通常显示出有限的底物范围。然而,电化学叠氮化被认为对环境友好且非常简单,同时还提供了一种高效的合成1,2-二叠氮化物衍生物的方法。此外,它们为轻松获得1,2-二胺提供了一个明智的选择,可用于将来的现代合成。
更新日期:2018-03-07
中文翻译:

烯烃的自由基重氮化:Cu / Fe / Mn催化和电化学支持
通过使用过渡金属通过自由基机理形成C-N 3键的合成途径可提供高效的叠氮化物产物,该产物可进一步用于许多转化中,以合成各种有价值的药用含氮化合物。报告的方法证明了化学计量的重金属和有毒试剂(以N 3为来源),通常显示出有限的底物范围。然而,电化学叠氮化被认为对环境友好且非常简单,同时还提供了一种高效的合成1,2-二叠氮化物衍生物的方法。此外,它们为轻松获得1,2-二胺提供了一个明智的选择,可用于将来的现代合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号