当前位置:
X-MOL 学术
›
Chem. Asian J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nature of Bonding in Bowl‐Like B36 Cluster Revisited: Concentric (6π+18π) Double Aromaticity and Reason for the Preference of a Hexagonal Hole in a Central Location
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2018-04-10 , DOI: 10.1002/asia.201800174 Rui Li 1 , Xue-Rui You 1 , Kang Wang 1 , Hua-Jin Zhai 1
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2018-04-10 , DOI: 10.1002/asia.201800174 Rui Li 1 , Xue-Rui You 1 , Kang Wang 1 , Hua-Jin Zhai 1
Affiliation
|
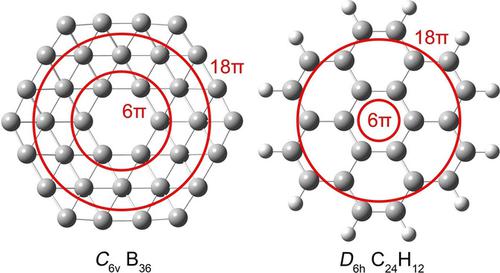
The bowl‐shaped C6v B36 cluster with a central hexagon hole is considered an ideal molecular model for low‐dimensional boron‐based nanosystems. Owing to the electron deficiency of boron, chemical bonding in the B36 cluster is intriguing, complicated, and has remained elusive despite a couple of papers in the literature. Herein, a bonding analysis is given through canonical molecular orbitals (CMOs) and adaptive natural density partitioning (AdNDP), further aided by natural bond orbital (NBO) analysis and orbital composition calculations. The concerted computational data establish the idea of concentric double π aromaticity for the B36 cluster, with inner 6π and outer 18π electron counting, which both conform to the (4n+2) Hückel rule. The updated bonding picture differs from existing knowledge of the system. A refined bonding model is also proposed for coronene, of which the B36 cluster is an inorganic analogue. It is further shown that concentric double π aromaticity in the B36 cluster is retained and spatially fixed, irrespective of the migration of the hexagonal hole; the latter process changes the system energetically. The hexagonal hole is a destabilizing factor for σ/π CMOs. The central hexagon hole affects substantially fewer CMOs, thus making the bowl‐shaped C6v B36 cluster the global minimum.
中文翻译:

重新研究碗状B36团簇的键合性质:同心(6π+18π)双重芳香性和中心位置偏向六角孔的原因
具有中心六边形孔的碗状C 6 v B 36团簇被认为是低维硼基纳米系统的理想分子模型。由于硼缺乏电子,B 36团簇中的化学键很有趣,很复杂,尽管有两篇文献发表,但仍然难以捉摸。在本文中,通过规范分子轨道(CMO)和自适应自然密度分配(AdNDP)进行键合分析,并进一步通过自然键合轨道(NBO)分析和轨道组成计算进行辅助。一致的计算数据为B 36建立了同心双π芳香度的概念团簇,内部具有6π和外部18π电子计数,均符合(4 n +2)Hückel规则。更新的绑定图片与系统的现有知识不同。还提出了一种用于日冕的改进的键合模型,其中B 36团簇是无机类似物。进一步表明,与六角孔的迁移无关,B 36团簇中的同心双π芳香性得以保留并在空间上固定。后面的过程将极大地改变系统。六角孔是σ/πCMO的不稳定因素。中心六边形孔影响的CMO少得多,因此使碗形C 6 v B 36簇成为全局最小值。
更新日期:2018-04-10
中文翻译:

重新研究碗状B36团簇的键合性质:同心(6π+18π)双重芳香性和中心位置偏向六角孔的原因
具有中心六边形孔的碗状C 6 v B 36团簇被认为是低维硼基纳米系统的理想分子模型。由于硼缺乏电子,B 36团簇中的化学键很有趣,很复杂,尽管有两篇文献发表,但仍然难以捉摸。在本文中,通过规范分子轨道(CMO)和自适应自然密度分配(AdNDP)进行键合分析,并进一步通过自然键合轨道(NBO)分析和轨道组成计算进行辅助。一致的计算数据为B 36建立了同心双π芳香度的概念团簇,内部具有6π和外部18π电子计数,均符合(4 n +2)Hückel规则。更新的绑定图片与系统的现有知识不同。还提出了一种用于日冕的改进的键合模型,其中B 36团簇是无机类似物。进一步表明,与六角孔的迁移无关,B 36团簇中的同心双π芳香性得以保留并在空间上固定。后面的过程将极大地改变系统。六角孔是σ/πCMO的不稳定因素。中心六边形孔影响的CMO少得多,因此使碗形C 6 v B 36簇成为全局最小值。











































 京公网安备 11010802027423号
京公网安备 11010802027423号