当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium‐Catalyzed Enantioselective C−H Olefination of Diaryl Sulfoxides through Parallel Kinetic Resolution and Desymmetrization
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-03-23 , DOI: 10.1002/anie.201801146 Yu-Chao Zhu 1 , Yan Li 1 , Bo-Chao Zhang 1 , Feng-Xu Zhang 1 , Yi-Nuo Yang 1 , Xi-Sheng Wang 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-03-23 , DOI: 10.1002/anie.201801146 Yu-Chao Zhu 1 , Yan Li 1 , Bo-Chao Zhang 1 , Feng-Xu Zhang 1 , Yi-Nuo Yang 1 , Xi-Sheng Wang 1
Affiliation
|
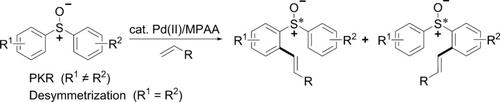
The first example of PdII‐catalyzed enantioselective C−H olefination with non‐chiral or racemic sulfoxides as directing groups was developed. A variety of chiral diaryl sulfoxides were synthesized with high enantioselectivity (up to 99 %) through both desymmetrization and parallel kinetic resolution (PKR). This is the first report of PdII‐catalyzed enantioselective C(sp2)−H functionalization through PKR, and it represents a novel strategy to construct sulfur chiral centers.
中文翻译:

二芳基亚砜通过平行动力学拆分和不对称化进行钯催化的对映选择性CH烯烃化
开发了第一个用非手性或外消旋亚砜作为导向基团的Pd II催化对映选择性CH烯化反应的例子。通过不对称化和平行动力学拆分(PKR)合成了具有高对映选择性(高达99%)的多种手性二芳基亚砜。这是Pd II催化通过PKR进行对映选择性C(sp 2)-H功能化的首次报道,它代表了构建硫手性中心的新策略。
更新日期:2018-03-23
中文翻译:

二芳基亚砜通过平行动力学拆分和不对称化进行钯催化的对映选择性CH烯烃化
开发了第一个用非手性或外消旋亚砜作为导向基团的Pd II催化对映选择性CH烯化反应的例子。通过不对称化和平行动力学拆分(PKR)合成了具有高对映选择性(高达99%)的多种手性二芳基亚砜。这是Pd II催化通过PKR进行对映选择性C(sp 2)-H功能化的首次报道,它代表了构建硫手性中心的新策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号