当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Activation of Triphenylphosphine Oxide Mediated by Trivalent Organouranium Species
Organometallics ( IF 2.5 ) Pub Date : 2018-03-06 00:00:00 , DOI: 10.1021/acs.organomet.7b00896 Caleb J. Tatebe 1 , Zhengjia Tong 1 , John J. Kiernicki 1 , Ezra J. Coughlin 1 , Matthias Zeller 1 , Suzanne C. Bart 1
Organometallics ( IF 2.5 ) Pub Date : 2018-03-06 00:00:00 , DOI: 10.1021/acs.organomet.7b00896 Caleb J. Tatebe 1 , Zhengjia Tong 1 , John J. Kiernicki 1 , Ezra J. Coughlin 1 , Matthias Zeller 1 , Suzanne C. Bart 1
Affiliation
|
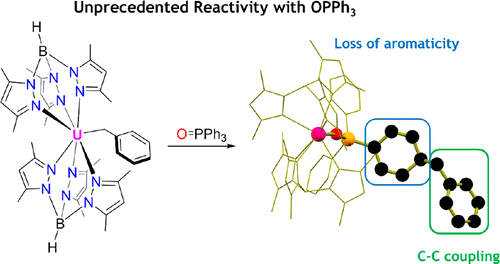
Treating a family of uranium benzyl compounds, Tp*2U(CH2Ph) (1-Bn), Tp*2U(CH2-para-iPrPh) (1-iPr), Tp*2U(CH2-para-tBuPh) (1-tBu), or Tp*2U(CH2-meta-OMePh) (1-OMe), which are supported by two hydrotris(3,5-dimethylpyrazolyl)borate (Tp*) ligands, with a single equivalent of triphenylphosphine oxide (OPPh3) causes a unique carbon–carbon coupling to occur. The products of this reaction, Tp*2U[OP(C6H5)2(C6H5CH2C6H5)] (2-Ph), Tp*2U[OP(C6H5)2(C6H5CH2-p-iPrC6H4)] (2-iPr), Tp*2U[OP(C6H5)2(C6H5CH2-p-tBuC6H4)] (2-tBu), and Tp*2U[OP(C6H5)2(C6H5CH2-m-OCH3C6H4)] (2-OMe), are characterized by coupling between the benzyl substituent and the para-carbon of one of the phenyl groups of OPPh3. To probe the scope of this unusual reactivity, 1-Bn was treated with different tris(aryl)phosphine oxides, including tris(p-tolyl)phosphine oxide, which yields Tp*2U[OP(p-tolyl)2(C6H4(CH3)CH2C6H5)] (3-tolyl). All compounds were characterized by multinuclear NMR, vibrational, and electronic absorption spectroscopies. When possible, X-ray diffraction was used to confirm molecular structures.
中文翻译:

三价有机铀物种介导的三苯基膦氧化物的活化
治疗家族铀苄基化合物的,TP * 2 U(CH 2 PH)(1-BN),TP * 2 U(CH 2 -对-我PrPh)(1-我镨),TP * 2 U(CH 2 -对位-吨BUPH)(1-吨卜),或TP * 2 U(CH 2 -元-OMePh)(1-OME),其由两个氢三(3,5-二甲基)硼酸盐支持(TP *)配体,单当量的三苯基氧化膦(OPPh 3)引起独特的碳-碳偶合发生。该反应的产物Tp * 2 U [OP(C 6 H 5)2(C 6 H 5 CH 2 C 6 H 5)](2-Ph),Tp * 2 U [OP(C 6 H 5)2(C 6 H 5 CH 2 - p - i PrC 6 H 4)](2- i Pr),Tp * 2 U [OP(C 6 H 5)2(C 6 H ^ 5 CH 2 - p -吨BUC 6 ħ 4)](2-吨卜),和Tp * 2 U [OP(C 6 H ^ 5)2(C 6 H ^ 5 CH 2 -米- OCH 3 C 6 H 4)](2-OMe)的特征在于,苄基取代基与OPPh 3的一个苯基的对位碳之间偶联。为了探究这种异常反应活性的范围,对1-Bn进行了不同的三(芳基)氧化膦处理,包括三(对甲苯基)氧化膦,生成的Tp * 2 U [OP(对甲苯基)2(C 6 H 4(CH 3)CH 2 C 6 H 5)](3-甲苯基)。所有化合物均通过多核NMR,振动和电子吸收光谱进行表征。如果可能,使用X射线衍射来确认分子结构。
更新日期:2018-03-07
中文翻译:

三价有机铀物种介导的三苯基膦氧化物的活化
治疗家族铀苄基化合物的,TP * 2 U(CH 2 PH)(1-BN),TP * 2 U(CH 2 -对-我PrPh)(1-我镨),TP * 2 U(CH 2 -对位-吨BUPH)(1-吨卜),或TP * 2 U(CH 2 -元-OMePh)(1-OME),其由两个氢三(3,5-二甲基)硼酸盐支持(TP *)配体,单当量的三苯基氧化膦(OPPh 3)引起独特的碳-碳偶合发生。该反应的产物Tp * 2 U [OP(C 6 H 5)2(C 6 H 5 CH 2 C 6 H 5)](2-Ph),Tp * 2 U [OP(C 6 H 5)2(C 6 H 5 CH 2 - p - i PrC 6 H 4)](2- i Pr),Tp * 2 U [OP(C 6 H 5)2(C 6 H ^ 5 CH 2 - p -吨BUC 6 ħ 4)](2-吨卜),和Tp * 2 U [OP(C 6 H ^ 5)2(C 6 H ^ 5 CH 2 -米- OCH 3 C 6 H 4)](2-OMe)的特征在于,苄基取代基与OPPh 3的一个苯基的对位碳之间偶联。为了探究这种异常反应活性的范围,对1-Bn进行了不同的三(芳基)氧化膦处理,包括三(对甲苯基)氧化膦,生成的Tp * 2 U [OP(对甲苯基)2(C 6 H 4(CH 3)CH 2 C 6 H 5)](3-甲苯基)。所有化合物均通过多核NMR,振动和电子吸收光谱进行表征。如果可能,使用X射线衍射来确认分子结构。











































 京公网安备 11010802027423号
京公网安备 11010802027423号