当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Separation of Dimethyl Carbonate and Methanol by Deep Eutectic Solvents: Liquid–Liquid Equilibrium Measurements and Thermodynamic Modeling
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-03-06 00:00:00 , DOI: 10.1021/acs.jced.7b00858 Xiaowei Liu 1 , Dongmei Xu 1 , Baotao Diao 1 , Jun Gao 1 , Lianzheng Zhang 1 , Yixin Ma 1 , Yinglong Wang 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-03-06 00:00:00 , DOI: 10.1021/acs.jced.7b00858 Xiaowei Liu 1 , Dongmei Xu 1 , Baotao Diao 1 , Jun Gao 1 , Lianzheng Zhang 1 , Yixin Ma 1 , Yinglong Wang 2
Affiliation
|
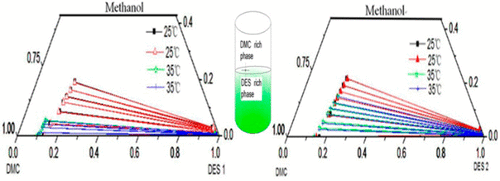
To separate the mixture of dimethyl carbonate and methanol, two deep eutectic solvents (DESs), choline chloride with glycerol and ethylene glycol, with the molar ratio of 1:2 were prepared to separate dimethyl carbonate from the mixture by liquid–liquid extraction. The liquid–liquid equilibrium (LLE) data for the ternary systems of dimethyl carbonate + methanol + DESs were determined at temperature of 298.15 and 308.15 K under 101.3 KPa. The measured tie-line data were validated by the Bachman equation, and the correlation coefficients (R2) were all close to 1. Meanwhile, the distribution coefficient and selectivity were calculated from the experimental results. The LLE experimental data were correlated using the nonrandom two-liquid (NRTL) model, and the correlated results agree well with the LLE experimental data. Also, the parameters of the NRTL model were regressed.
中文翻译:

深共熔溶剂分离碳酸二甲酯和甲醇:液-液相平衡测量和热力学模型
为了分离碳酸二甲酯和甲醇的混合物,准备了两种深度低共熔溶剂(DESs),氯化胆碱与甘油和乙二醇,摩尔比为1:2,可以通过液-液萃取从混合物中分离出碳酸二甲酯。在101.3 KPa下,在298.15和308.15 K的温度下测定了碳酸二甲酯+甲醇+ DESs三元体系的液-液平衡(LLE)数据。测得的联络线数据通过Bachman方程进行验证,并且相关系数为(R 2)均接近于1。同时,根据实验结果计算了分布系数和选择性。使用非随机两液(NRTL)模型对LLE实验数据进行了相关,并且相关结果与LLE实验数据非常吻合。此外,NRTL模型的参数也进行了回归。
更新日期:2018-03-06
中文翻译:

深共熔溶剂分离碳酸二甲酯和甲醇:液-液相平衡测量和热力学模型
为了分离碳酸二甲酯和甲醇的混合物,准备了两种深度低共熔溶剂(DESs),氯化胆碱与甘油和乙二醇,摩尔比为1:2,可以通过液-液萃取从混合物中分离出碳酸二甲酯。在101.3 KPa下,在298.15和308.15 K的温度下测定了碳酸二甲酯+甲醇+ DESs三元体系的液-液平衡(LLE)数据。测得的联络线数据通过Bachman方程进行验证,并且相关系数为(R 2)均接近于1。同时,根据实验结果计算了分布系数和选择性。使用非随机两液(NRTL)模型对LLE实验数据进行了相关,并且相关结果与LLE实验数据非常吻合。此外,NRTL模型的参数也进行了回归。











































 京公网安备 11010802027423号
京公网安备 11010802027423号