当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic insights into the different chemoselectivities of Rh2(ii)-catalyzed ring expansion of cyclobutanol-substituted aryl azides and C–H bond amination of cyclopentanol-substituted aryl azides: a DFT study†
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-03-06 00:00:00 , DOI: 10.1039/c8qo00113h Dafang Gao 1, 2, 3, 4, 5 , Xiaoguang Bao 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-03-06 00:00:00 , DOI: 10.1039/c8qo00113h Dafang Gao 1, 2, 3, 4, 5 , Xiaoguang Bao 1, 2, 3, 4, 5
Affiliation
|
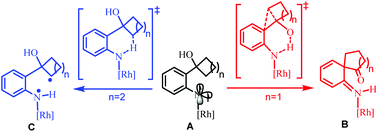
The mechanism of Rh2(II)-catalyzed ring expansion of ortho-cyclobutanol-substituted aryl azides to access N-heterocycles was investigated by DFT calculations. After the generation of the key Rh2(II)-N-arylnitrene intermediate (A), computational results suggest that it is more feasible to undergo proton transfer from the O–H group of the ortho-cyclobutanol moiety to the nitrene site in the singlet state, along with four-membered ring expansion via [1,2] migration in a concerted manner, to form the key intermediate (B). Subsequently, the [1,3] migration step driven by rearomatization could follow to yield the final benzazepinone product. For the substrate, ortho-cyclopentanol-substituted aryl azide, H-atom abstraction (HAA) from the proximal sp3 C–H bond in the triplet state is more favorable than competitive HAA/proton transfer from the O–H group after the formation of the rhodium N-aryl nitrene intermediate. Subsequently, the C–H bond amination product, indoline, could be obtained via a radical rebound step. The origin of the different chemoselectivities for the ortho-cyclobutanol-substituted and ortho-cyclopentanol-substituted aryl azides was discussed.
中文翻译:

对Rh 2(ii)催化的环丁醇取代的芳基叠氮化物的环扩环和环戊醇取代的芳基叠氮化物的CH键胺化的不同化学选择性的力学见解:DFT研究†
通过DFT计算研究了Rh 2(II)催化的邻-环丁醇取代的芳基叠氮化物扩环进入N-杂环的机理。在生成关键的Rh 2(II)-N-芳基亚硝基中间体(A)后,计算结果表明,将质子从邻环丁醇部分的O-H基团转移到苯环中的腈位置更可行。单重态以及通过[1,2]迁移以协同方式进行的四元环扩展,形成关键中间体(B)。随后,由重金属化驱动的[1,3]迁移步骤可继而产生最终的苯并ze庚酮产品。对于底物,邻位环戊醇取代的芳基叠氮化物,从三重态sp 3 C–H近端的H原子抽象(HAA)比形成后从O–H组进行的竞争性HAA /质子转移更有利N-芳基铑亚硝基铑中间体。随后,可以通过自由基反弹步骤获得C–H键的胺化产物二氢吲哚。讨论了邻-环丁醇取代的和邻-环戊醇取代的芳基叠氮化物不同化学选择性的起源。
更新日期:2018-03-06
中文翻译:

对Rh 2(ii)催化的环丁醇取代的芳基叠氮化物的环扩环和环戊醇取代的芳基叠氮化物的CH键胺化的不同化学选择性的力学见解:DFT研究†
通过DFT计算研究了Rh 2(II)催化的邻-环丁醇取代的芳基叠氮化物扩环进入N-杂环的机理。在生成关键的Rh 2(II)-N-芳基亚硝基中间体(A)后,计算结果表明,将质子从邻环丁醇部分的O-H基团转移到苯环中的腈位置更可行。单重态以及通过[1,2]迁移以协同方式进行的四元环扩展,形成关键中间体(B)。随后,由重金属化驱动的[1,3]迁移步骤可继而产生最终的苯并ze庚酮产品。对于底物,邻位环戊醇取代的芳基叠氮化物,从三重态sp 3 C–H近端的H原子抽象(HAA)比形成后从O–H组进行的竞争性HAA /质子转移更有利N-芳基铑亚硝基铑中间体。随后,可以通过自由基反弹步骤获得C–H键的胺化产物二氢吲哚。讨论了邻-环丁醇取代的和邻-环戊醇取代的芳基叠氮化物不同化学选择性的起源。











































 京公网安备 11010802027423号
京公网安备 11010802027423号