当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The role of oxygen vacancies in biomass deoxygenation by reducible zinc/zinc oxide catalysts†
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-03-06 00:00:00 , DOI: 10.1039/c7cy02535a Xiao Xiao 1, 2, 3, 4, 5 , Helen Bergstrom 1, 2, 3 , Ryan Saenger 1, 2, 3 , Benjamin Johnson 1, 2, 3 , Runcang Sun 4, 5, 6, 7 , Andrew Peterson 1, 2, 3
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-03-06 00:00:00 , DOI: 10.1039/c7cy02535a Xiao Xiao 1, 2, 3, 4, 5 , Helen Bergstrom 1, 2, 3 , Ryan Saenger 1, 2, 3 , Benjamin Johnson 1, 2, 3 , Runcang Sun 4, 5, 6, 7 , Andrew Peterson 1, 2, 3
Affiliation
|
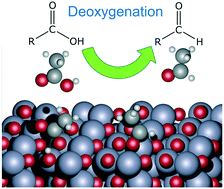
Selective removal of oxygen is the key challenge in the upgrading of biomass-derived molecules, and reducible metal oxides have shown the ability to catalytically remove oxygen even at low exogenous H2 pressures. As opposed to a traditional surface reaction, it is possible that oxygen vacancies provide the active sites in such catalysts, in a Mars–van Krevelen (MvK)-like mechanism. In this work, we use a combination of theoretical calculations and experimental measurements to provide conclusive evidence that this reaction proceeds through such a vacancy-driven mechanism, for the example system of acetic acid deoxygenation to acetaldehyde over reducible zinc oxide surfaces. Density functional theory (DFT) calculations suggest that the catalyst without an oxygen vacancy on the surface was relatively unreactive, due to a strong energetic penalty of breaking the C–O bond, while the presence of the vacancy provides the reducing power to facilitate this elementary step. Experimentally, to examine the role of vacancies directly, we compared the rates and selectivities of acetic acid deoxygenation for standard hydrodeoxygenation conditions with rates in H2-free conditions starting with reduced Zn metal, in which presumably the only active sites are vacancies created through diffusion. We found a striking similarity in the product distribution, suggesting a common mechanism in both cases. Specifically, metallic zinc with numerous vacant sites on the surface showed a high activity in promoting the deoxygenation reaction, while oxidized zinc (without oxygen vacancies) was fully deactivated. This study suggests that a unique vacancy-driven mechanism is responsible for the reactivity of reducible metal oxide catalysts.
中文翻译:

氧空位在可还原锌/氧化锌催化剂对生物质脱氧中的作用†
选择性去除氧气是生物质衍生分子升级的关键挑战,而且可还原的金属氧化物显示出即使在低外源H 2下也能催化去除氧气的能力。压力。与传统的表面反应相反,氧空位可能以类似Mars–van Krevelen(MvK)的机制提供此类催化剂中的活性位点。在这项工作中,我们将理论计算和实验测量相结合以提供确凿的证据,表明该反应是通过这种空位驱动的机理进行的,例如在可还原氧化锌表面上将乙酸脱氧为乙醛的系统。密度泛函理论(DFT)的计算表明,由于破坏C–O键的高能损失,表面无氧空位的催化剂相对没有反应性,而空位的存在提供了促进这种基本元素还原的能力。步。通过实验,直接检查职位空缺的作用,不含2的条件始于还原的Zn金属,其中大概唯一的活性位点是通过扩散产生的空位。我们发现产品分布上的惊人相似之处,表明这两种情况下的共同机制。具体地,表面上具有许多空位的金属锌在促进脱氧反应中显示出高活性,而氧化锌(无氧空位)被完全失活。这项研究表明,独特的空位驱动机制负责还原性金属氧化物催化剂的反应性。
更新日期:2018-03-06
中文翻译:

氧空位在可还原锌/氧化锌催化剂对生物质脱氧中的作用†
选择性去除氧气是生物质衍生分子升级的关键挑战,而且可还原的金属氧化物显示出即使在低外源H 2下也能催化去除氧气的能力。压力。与传统的表面反应相反,氧空位可能以类似Mars–van Krevelen(MvK)的机制提供此类催化剂中的活性位点。在这项工作中,我们将理论计算和实验测量相结合以提供确凿的证据,表明该反应是通过这种空位驱动的机理进行的,例如在可还原氧化锌表面上将乙酸脱氧为乙醛的系统。密度泛函理论(DFT)的计算表明,由于破坏C–O键的高能损失,表面无氧空位的催化剂相对没有反应性,而空位的存在提供了促进这种基本元素还原的能力。步。通过实验,直接检查职位空缺的作用,不含2的条件始于还原的Zn金属,其中大概唯一的活性位点是通过扩散产生的空位。我们发现产品分布上的惊人相似之处,表明这两种情况下的共同机制。具体地,表面上具有许多空位的金属锌在促进脱氧反应中显示出高活性,而氧化锌(无氧空位)被完全失活。这项研究表明,独特的空位驱动机制负责还原性金属氧化物催化剂的反应性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号