当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Adsorption of PAHs on interstellar ice viewed by classical molecular dynamics†
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-03-06 00:00:00 , DOI: 10.1039/c8cp00593a Eric Michoulier 1, 2, 3, 4 , Jennifer A. Noble 1, 2, 3, 4, 5 , Aude Simon 4, 6, 7 , Joëlle Mascetti 4, 6, 7 , Céline Toubin 1, 2, 3, 4
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-03-06 00:00:00 , DOI: 10.1039/c8cp00593a Eric Michoulier 1, 2, 3, 4 , Jennifer A. Noble 1, 2, 3, 4, 5 , Aude Simon 4, 6, 7 , Joëlle Mascetti 4, 6, 7 , Céline Toubin 1, 2, 3, 4
Affiliation
|
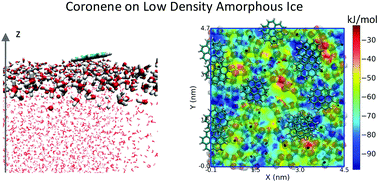
Polycyclic Aromatic Hydrocarbons (PAHs) are a family of molecules which represent the best candidates to explain the observation of one set of features in the Interstellar Medium (ISM): the Aromatic Interstellar Bands (AIBs). They could also contribute to the Diffuse Interstellar Bands (DIBs). In dense molecular clouds, PAHs may condense onto interstellar grains, contributing to the complex chemistry occurring in their icy mantles, composed essentially of water. In this context, the adsorption of various aromatic molecules, from benzene to ovalene, on different ices – both amorphous and crystalline – is investigated by means of classical molecular dynamics simulations. Initially, a systematic parametrization of the electronic charges on the chosen PAHs in these environments is carried out, and benchmarked with reference to free energies of solvation in liquid water. Then we propose a new, rigorous methodology, transferable to any other PAH or molecular species, to evaluate the charges to be applied to the molecule in the gas phase, at interfaces, or in liquid water. Ultimately, the adsorption energies calculated for the various PAHs are used to derive a function predicting the adsorption energy of any PAH on a given ice surface as a function of the number of C and H atoms it contains. For all PAHs studied, the largest adsorption energies are found on the crystalline hexagonal ice surface (Ih). Binding energy maps constructed for each PAH–ice pair give valuable insight into adsorption site densities and the barriers to surface diffusion. One key result is that the amorphous surface offers a smaller number of adsorption sites compared to that of hexagonal ice. A direct correlation between the location of energetically favourable adsorption sites and the presence of dangling H-bonds is also demonstrated using these maps, showing that PAHs adsorb preferentially on sites offering dangling H-bonds. The present work represents a complete description of PAH–ice interaction in the ground electronic state and at low temperature, providing the binding energies and barrier heights necessary to the ongoing improvement of astrochemical models.
中文翻译:

经典分子动力学观察到星际冰上多环芳烃的吸附†
多环芳香烃(PAH)是代表解释星际介质(ISM)中一组特征的观测的最佳候选分子:芳香星际带(AIB)。它们还可以为弥漫星际频带(DIB)做出贡献。在致密的分子云中,多环芳烃可能凝结在星际颗粒上,从而导致在其冰冷的地幔中发生的复杂化学反应(主要由水组成)。在这种情况下,通过经典的分子动力学模拟研究了从苯到椭圆烯的各种芳香分子在不同冰块上(非晶态和结晶态)的吸附。最初,在这些环境中对所选PAH上的电荷进行了系统的参数化,并以液态水中的溶剂化自由能为基准。然后,我们提出了一种新的,严格的方法,可以转移到任何其他PAH或分子种类中,以评估要在气相,界面或液态水中应用于分子的电荷。最终,为各种PAH计算的吸附能被用来推导一个函数,该函数根据给定的冰表面上所含C和H原子的数量来预测给定冰面上任何PAH的吸附能。对于所有研究的多环芳烃,在晶体六角形冰面(Ih)上发现了最大的吸附能。为每个PAH-冰对构建的结合能图可提供有关吸附位点密度和表面扩散障碍的宝贵见解。一个关键的结果是,与六角形冰相比,无定形表面提供的吸附点数量更少。使用这些图还证明了在能量上有利的吸附位点的位置和悬挂的H键之间的直接相关性,表明PAHs优先吸附在提供悬挂的H键的位置上。本工作代表了在地面电子状态和低温下PAH-冰相互作用的完整描述,提供了不断改进的星化模型所必需的结合能和势垒高度。表明PAHs优先吸附在提供悬空H键的位点上。本工作代表了在地面电子状态和低温下PAH-冰相互作用的完整描述,提供了不断改进的星化模型所必需的结合能和势垒高度。表明PAHs优先吸附在提供悬空H键的位点上。本工作代表了在地面电子状态和低温下PAH-冰相互作用的完整描述,提供了不断改进的星化模型所必需的结合能和势垒高度。
更新日期:2018-03-06
中文翻译:

经典分子动力学观察到星际冰上多环芳烃的吸附†
多环芳香烃(PAH)是代表解释星际介质(ISM)中一组特征的观测的最佳候选分子:芳香星际带(AIB)。它们还可以为弥漫星际频带(DIB)做出贡献。在致密的分子云中,多环芳烃可能凝结在星际颗粒上,从而导致在其冰冷的地幔中发生的复杂化学反应(主要由水组成)。在这种情况下,通过经典的分子动力学模拟研究了从苯到椭圆烯的各种芳香分子在不同冰块上(非晶态和结晶态)的吸附。最初,在这些环境中对所选PAH上的电荷进行了系统的参数化,并以液态水中的溶剂化自由能为基准。然后,我们提出了一种新的,严格的方法,可以转移到任何其他PAH或分子种类中,以评估要在气相,界面或液态水中应用于分子的电荷。最终,为各种PAH计算的吸附能被用来推导一个函数,该函数根据给定的冰表面上所含C和H原子的数量来预测给定冰面上任何PAH的吸附能。对于所有研究的多环芳烃,在晶体六角形冰面(Ih)上发现了最大的吸附能。为每个PAH-冰对构建的结合能图可提供有关吸附位点密度和表面扩散障碍的宝贵见解。一个关键的结果是,与六角形冰相比,无定形表面提供的吸附点数量更少。使用这些图还证明了在能量上有利的吸附位点的位置和悬挂的H键之间的直接相关性,表明PAHs优先吸附在提供悬挂的H键的位置上。本工作代表了在地面电子状态和低温下PAH-冰相互作用的完整描述,提供了不断改进的星化模型所必需的结合能和势垒高度。表明PAHs优先吸附在提供悬空H键的位点上。本工作代表了在地面电子状态和低温下PAH-冰相互作用的完整描述,提供了不断改进的星化模型所必需的结合能和势垒高度。表明PAHs优先吸附在提供悬空H键的位点上。本工作代表了在地面电子状态和低温下PAH-冰相互作用的完整描述,提供了不断改进的星化模型所必需的结合能和势垒高度。











































 京公网安备 11010802027423号
京公网安备 11010802027423号