Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2018-03-06 , DOI: 10.1016/j.bmcl.2018.02.052 Jie Zhang , Tatsunori Sasaki , Wei Li , Kazuya Nagata , Koji Higai , Feng Feng , Jian Wang , Maosheng Cheng , Kazuo Koike
|
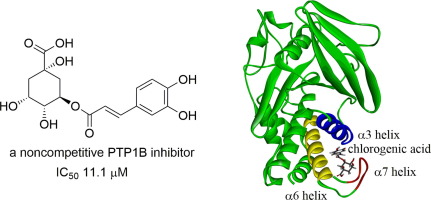
Considerable attention has been paid to protein tyrosine phosphatase 1B (PTP1B) inhibitors as a potential therapy for diabetes, obesity, and cancer. Ten caffeoylquinic acid derivatives (1–10) from leaves of Artemisia princeps Pamp. (Asteraceae) were identified as natural PTP1B inhibitors. Among them, chlorogenic acid (3) showed the most potent inhibitory activity (IC50 11.1 μM). Compound 3 was demonstrated to be a noncompetitive inhibitor by a kinetic analysis. Molecular docking simulation suggested that compound 3 bound to the allosteric site of PTP1B. Furthermore, compound 3 showed remarkable selectivity against four homologous PTPs. According to these findings, compound 3 might be potentially valuable for further drug development.
中文翻译:

鉴定咖啡因奎宁酸衍生物为蒿蒿的天然蛋白质酪氨酸磷酸酶1B抑制剂
人们已经非常重视蛋白质酪氨酸磷酸酶1B(PTP1B)抑制剂作为糖尿病,肥胖症和癌症的潜在疗法。十种蒿蒿叶片中的十种咖啡酰奎尼酸衍生物(1 – 10)。(菊科)被鉴定为天然PTP1B抑制剂。其中,绿原酸(3)表现出最强的抑制活性(IC 50 11.1μM)。通过动力学分析证明化合物3是非竞争性抑制剂。分子对接模拟表明化合物3绑定到PTP1B的变构位点。此外,化合物3对四个同源PTP表现出显着的选择性。根据这些发现,化合物3对于进一步的药物开发可能具有潜在的价值。









































 京公网安备 11010802027423号
京公网安备 11010802027423号