当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Superoxide Stabilization and a Universal KO2 Growth Mechanism in Potassium–Oxygen Batteries
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-03-23 , DOI: 10.1002/anie.201801344 Wanwan Wang 1 , Nien-Chu Lai 1 , Zhuojian Liang 1 , Yu Wang 1 , Yi-Chun Lu 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-03-23 , DOI: 10.1002/anie.201801344 Wanwan Wang 1 , Nien-Chu Lai 1 , Zhuojian Liang 1 , Yu Wang 1 , Yi-Chun Lu 1
Affiliation
|
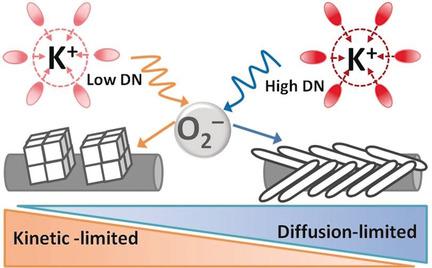
Rechargeable potassium–oxygen (K‐O2) batteries promise to provide higher round‐trip efficiency and cycle life than other alkali–oxygen batteries with satisfactory gravimetric energy density (935 Wh kg−1). Exploiting a strong electron‐donating solvent, for example, dimethyl sulfoxide (DMSO) strongly stabilizes the discharge product (KO2), resulting in significant improvement in electrode kinetics and chemical/electrochemical reversibility. The first DMSO‐based K‐O2 battery demonstrates a much higher energy efficiency and stability than the glyme‐based electrolyte. A universal KO2 growth model is developed and it is demonstrated that the ideal solvent for K‐O2 batteries should strongly stabilize superoxide (strong donor ability) to obtain high electrode kinetics and reversibility while providing fast oxygen diffusion to achieve high discharge capacity. This work elucidates key electrolyte properties that control the efficiency and reversibility of K‐O2 batteries.
中文翻译:

钾氧电池中的超氧化物稳定化和普遍的KO2生长机制
与具有令人满意的重量能量密度(935 Wh kg -1)的其他碱性氧气电池相比,可充电的钾氧气电池(K-O 2)有望提供更高的往返效率和循环寿命。使用强供电子溶剂(例如二甲亚砜(DMSO))可以使放电产物(KO 2)稳定,从而显着改善电极动力学和化学/电化学可逆性。第一个基于DMSO的K‐O 2电池比基于甘醇二甲醚的电解质具有更高的能源效率和稳定性。建立了通用的KO 2生长模型,并证明了K‐O 2的理想溶剂电池应强烈稳定超氧化物(强大的供体能力)以获得高电极动力学和可逆性,同时提供快速的氧气扩散以实现高放电容量。这项工作阐明了控制K‐O 2电池效率和可逆性的关键电解质性能。
更新日期:2018-03-23
中文翻译:

钾氧电池中的超氧化物稳定化和普遍的KO2生长机制
与具有令人满意的重量能量密度(935 Wh kg -1)的其他碱性氧气电池相比,可充电的钾氧气电池(K-O 2)有望提供更高的往返效率和循环寿命。使用强供电子溶剂(例如二甲亚砜(DMSO))可以使放电产物(KO 2)稳定,从而显着改善电极动力学和化学/电化学可逆性。第一个基于DMSO的K‐O 2电池比基于甘醇二甲醚的电解质具有更高的能源效率和稳定性。建立了通用的KO 2生长模型,并证明了K‐O 2的理想溶剂电池应强烈稳定超氧化物(强大的供体能力)以获得高电极动力学和可逆性,同时提供快速的氧气扩散以实现高放电容量。这项工作阐明了控制K‐O 2电池效率和可逆性的关键电解质性能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号