当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium/Norbornene‐Catalyzed C−H Alkylation/Alkyne Insertion/Indole Dearomatization Domino Reaction: Assembly of Spiroindolenine‐Containing Pentacyclic Frameworks
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-03-23 , DOI: 10.1002/anie.201801894 Lu Bai 1 , Jingjing Liu 1 , Wenjie Hu 1 , Kunyu Li 1 , Yaoyu Wang 1 , Xinjun Luan 1, 2
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-03-23 , DOI: 10.1002/anie.201801894 Lu Bai 1 , Jingjing Liu 1 , Wenjie Hu 1 , Kunyu Li 1 , Yaoyu Wang 1 , Xinjun Luan 1, 2
Affiliation
|
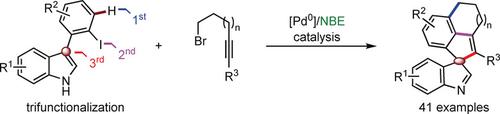
Reported is a highly chemoselective intermolecular annulation of indole‐based biaryls with bromoalkyl alkynes by using palladium/norbornene (Pd/NBE) cooperative catalysis. This reaction is realized through a sequence of Catellani‐type C−H alkylation, alkyne insertion, and indole dearomatization, by forming two C(sp2)−C(sp3) and one C(sp2)−C(sp2) bonds in a single chemical operation, thus providing a diverse range of pentacyclic molecules, containing a spiroindolenine fragment, in good yields with excellent functional‐group tolerance. Preliminary mechanistic studies reveal that C−H bond cleavage is likely involved in the rate‐determining step, and the indole dearomatization might take place through an olefin coordination/insertion and β‐hydride elimination Heck‐type pathway.
中文翻译:

钯/降冰片烯催化的CH烷基化/炔烃插入/吲哚脱芳香化反应
据报道,通过使用钯/降冰片烯(Pd / NBE)协同催化,吲哚基联芳基与溴代烷基炔烃具有高度化学选择性的分子间环化。通过形成两个C(sp 2)-C(sp 3)和一个C(sp 2)-C(sp 2)来实现一系列Catellani型CH烷基化,炔烃插入和吲哚脱芳香化反应)在一个化学操作中键合,从而以良好的收率和优异的官能团耐受性提供了多种范围的包含螺环吲哚片段的五环分子。初步的机理研究表明,速率决定步骤中可能涉及到CH键的断裂,并且吲哚脱芳香化作用可能是通过烯烃配位/插入和β-氢化物消除的Heck型途径进行的。
更新日期:2018-03-23
中文翻译:

钯/降冰片烯催化的CH烷基化/炔烃插入/吲哚脱芳香化反应
据报道,通过使用钯/降冰片烯(Pd / NBE)协同催化,吲哚基联芳基与溴代烷基炔烃具有高度化学选择性的分子间环化。通过形成两个C(sp 2)-C(sp 3)和一个C(sp 2)-C(sp 2)来实现一系列Catellani型CH烷基化,炔烃插入和吲哚脱芳香化反应)在一个化学操作中键合,从而以良好的收率和优异的官能团耐受性提供了多种范围的包含螺环吲哚片段的五环分子。初步的机理研究表明,速率决定步骤中可能涉及到CH键的断裂,并且吲哚脱芳香化作用可能是通过烯烃配位/插入和β-氢化物消除的Heck型途径进行的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号