当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An Iron Complex with a Bent, O‐Coordinated CO2 Ligand Discovered by Femtosecond Mid‐Infrared Spectroscopy
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-03-23 , DOI: 10.1002/anie.201800672 Steffen Straub 1 , Paul Brünker 1 , Jörg Lindner 1 , Peter Vöhringer 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-03-23 , DOI: 10.1002/anie.201800672 Steffen Straub 1 , Paul Brünker 1 , Jörg Lindner 1 , Peter Vöhringer 1
Affiliation
|
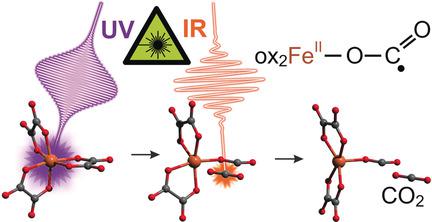
The activation of carbon dioxide by transition metals is widely recognized as a key step for utilizing this greenhouse gas as a renewable feedstock for the sustainable production of fine chemicals. However, the dynamics of CO2 binding and unbinding to and from the ligand sphere of a metal have never been observed in the time domain. The ferrioxalate anion is used in aqueous solution as a unique model system for these dynamics and femtosecond UV‐pump mid‐infrared‐probe spectroscopy is applied to explore its photoinduced primary processes in a time‐resolved fashion. Following optical excitation, a neutral CO2 molecule is expelled from the complex within about 500 fs to generate a highly intriguing pentacoordinate ferrous dioxalate that carries a bent carbon dioxide radical anion ligand, that is, a reductively activated form of CO2, which is end‐on‐coordinated to the metal center by one of its two oxygen atoms.
中文翻译:

飞秒中红外光谱法发现具有弯曲的,O配位的CO2配体的铁配合物
过渡金属对二氧化碳的活化被广泛认为是将这种温室气体用作可再生原料以可持续生产精细化学品的关键步骤。然而,从未在时域中观察到CO 2与金属的配体球结合和脱结合的动力学。铁氧体酸根阴离子在水溶液中用作这些动力学的独特模型系统,飞秒紫外泵浦中红外探针光谱法用于以时间分辨的方式探索其光诱导的主要过程。光激发后,中性CO 2分子在约500 fs内从复合物中排出,生成高度吸引人的五配位二草酸亚铁盐,其带有弯曲的二氧化碳自由基阴离子配体,即CO 2的还原活化形式,其端基与金属中心配位由它的两个氧原子之一。
更新日期:2018-03-23
中文翻译:

飞秒中红外光谱法发现具有弯曲的,O配位的CO2配体的铁配合物
过渡金属对二氧化碳的活化被广泛认为是将这种温室气体用作可再生原料以可持续生产精细化学品的关键步骤。然而,从未在时域中观察到CO 2与金属的配体球结合和脱结合的动力学。铁氧体酸根阴离子在水溶液中用作这些动力学的独特模型系统,飞秒紫外泵浦中红外探针光谱法用于以时间分辨的方式探索其光诱导的主要过程。光激发后,中性CO 2分子在约500 fs内从复合物中排出,生成高度吸引人的五配位二草酸亚铁盐,其带有弯曲的二氧化碳自由基阴离子配体,即CO 2的还原活化形式,其端基与金属中心配位由它的两个氧原子之一。









































 京公网安备 11010802027423号
京公网安备 11010802027423号