当前位置:
X-MOL 学术
›
ChemPlusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nucleophilic Cleavage of Lignin Model Compounds under Acidic Conditions in an Ionic Liquid: A Mechanistic Study.
ChemPlusChem ( IF 3.0 ) Pub Date : 2018-04-06 , DOI: 10.1002/cplu.201700486 William E S Hart 1 , Leigh Aldous 1, 2 , Jason B Harper 1
ChemPlusChem ( IF 3.0 ) Pub Date : 2018-04-06 , DOI: 10.1002/cplu.201700486 William E S Hart 1 , Leigh Aldous 1, 2 , Jason B Harper 1
Affiliation
|
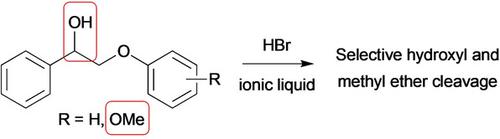
A range of lignin model compounds were examined for their reactivity with hydrogen bromide in the ionic liquid N-butylpyridinium triflate. It was found that the ionic liquid enabled rapid reaction at both the hydroxy and methyl ether sites of the model compounds at room temperature. Reactions at the phenyl ether moieties were more complex; rather than facilitating cleavage at these sites, alternate breakdown products that had not been seen in previous studies were observed; these products are consistent with functionalisation of the aromatic components of the model compounds.
中文翻译:

木质素模型化合物在酸性条件下在离子液体中的亲核裂解:机理研究。
检查了一系列木质素模型化合物在离子液体三氟甲磺酸N-丁基吡啶鎓中与溴化氢的反应性。发现在室温下离子液体能够在模型化合物的羟基和甲基醚位点处快速反应。苯基醚部分的反应更复杂;而不是促进这些位点的裂解,观察到了先前研究中未发现的其他分解产物;这些产物与模型化合物的芳族成分的功能化相一致。
更新日期:2018-04-06
中文翻译:

木质素模型化合物在酸性条件下在离子液体中的亲核裂解:机理研究。
检查了一系列木质素模型化合物在离子液体三氟甲磺酸N-丁基吡啶鎓中与溴化氢的反应性。发现在室温下离子液体能够在模型化合物的羟基和甲基醚位点处快速反应。苯基醚部分的反应更复杂;而不是促进这些位点的裂解,观察到了先前研究中未发现的其他分解产物;这些产物与模型化合物的芳族成分的功能化相一致。











































 京公网安备 11010802027423号
京公网安备 11010802027423号