当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Quantitative Characterization of the Membrane Dynamics of Newly Delivered TGF-β Receptors by Single-Molecule Imaging
Analytical Chemistry ( IF 6.7 ) Pub Date : 2018-03-06 00:00:00 , DOI: 10.1021/acs.analchem.7b03448 Mingliang Zhang 1, 2 , Zhen Zhang 2, 3 , Kangmin He 1, 2 , Jimin Wu 1 , Nan Li 2, 3 , Rong Zhao 2, 3 , Jinghe Yuan 2, 3 , Han Xiao 1 , Youyi Zhang 1 , Xiaohong Fang 2, 3
Analytical Chemistry ( IF 6.7 ) Pub Date : 2018-03-06 00:00:00 , DOI: 10.1021/acs.analchem.7b03448 Mingliang Zhang 1, 2 , Zhen Zhang 2, 3 , Kangmin He 1, 2 , Jimin Wu 1 , Nan Li 2, 3 , Rong Zhao 2, 3 , Jinghe Yuan 2, 3 , Han Xiao 1 , Youyi Zhang 1 , Xiaohong Fang 2, 3
Affiliation
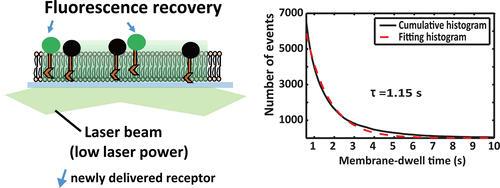
|
The dynamics and stoichiometry of receptors newly delivered on the plasma membrane play a vital role in cell signal transduction, yet knowledge of this process is limited because of the lack of suitable analytical methods. Here we developed a new strategy that combines single-molecule imaging (SMI) and fluorescence recovery after photobleaching (FRAP), named FRAP-SMI, to monitor and quantify individual newly delivered and inserted transmembrane receptors on plasma membranes of living cells. Transforming-growth-factor-β type II receptor (TβRII), a typical serine/threoninekinase receptor, was studied with this method. We first eliminated the fluorescence signals from the pre-existing EGFP-labeled TβRII molecules on the plasma membrane, and then we recorded the individual newly appeared TβRII-GFP by total-internal-reflection fluorescence imaging. The fluorescence-intensity distributions, photobleaching steps, and diffusion rates of the single TβRII-GFP molecules were analyzed. We reported, for the first time, that TβRII was transported to the plasma membrane mainly in the monomeric form in both resting and TGF-β1stimulated cells. This strongly supported our former discovery that TβRII could exist as a monomer on the cell membrane. We also found that ligand stimulation resulted in enhanced delivery rates and prolonged membrane-association times for the TβRII molecules. On the basis of these observations, we proposed a mechanism of TGF-β1-induced TβRII dimerization for receptor activation. Our method provides a useful tool for the real-time quantification of the spatial arrangement, mobility, and oligomerization of cell-surface proteins in living cells, thus providing a better understanding of cell signaling.
中文翻译:

通过单分子成像定量表征的新交付的TGF-β受体的膜动力学。
新传递到质膜上的受体的动力学和化学计量在细胞信号转导中起着至关重要的作用,但是由于缺乏合适的分析方法,对该过程的了解受到限制。在这里,我们开发了一种新的策略,将单分子成像(SMI)和光漂白后的荧光恢复(FRAP)结合起来,称为FRAP-SMI,以监视和量化活细胞质膜上的各个新递送和插入的跨膜受体。用这种方法研究了典型的丝氨酸/苏氨酸激酶受体转化生长因子βII型受体(TβRII)。我们首先从质膜上已存在的EGFP标记的TβRII分子中消除了荧光信号,然后通过全内反射荧光成像记录了新出现的单个TβRII-GFP。分析了单个TβRII-GFP分子的荧光强度分布,光漂白步骤和扩散速率。我们首次报道,在静止和TGF-β1刺激的细胞中,TβRII主要以单体形式转运至质膜。这有力地支持了我们以前的发现,即TβRII可以作为单体存在于细胞膜上。我们还发现,配体刺激导致TβRII分子的传递速率提高和膜缔合时间延长。基于这些观察,我们提出了TGF-β1诱导的TβRII二聚化激活受体的机制。我们的方法为实时定量活细胞中细胞表面蛋白的空间排列,迁移率和寡聚化提供了有用的工具,
更新日期:2018-03-06
中文翻译:

通过单分子成像定量表征的新交付的TGF-β受体的膜动力学。
新传递到质膜上的受体的动力学和化学计量在细胞信号转导中起着至关重要的作用,但是由于缺乏合适的分析方法,对该过程的了解受到限制。在这里,我们开发了一种新的策略,将单分子成像(SMI)和光漂白后的荧光恢复(FRAP)结合起来,称为FRAP-SMI,以监视和量化活细胞质膜上的各个新递送和插入的跨膜受体。用这种方法研究了典型的丝氨酸/苏氨酸激酶受体转化生长因子βII型受体(TβRII)。我们首先从质膜上已存在的EGFP标记的TβRII分子中消除了荧光信号,然后通过全内反射荧光成像记录了新出现的单个TβRII-GFP。分析了单个TβRII-GFP分子的荧光强度分布,光漂白步骤和扩散速率。我们首次报道,在静止和TGF-β1刺激的细胞中,TβRII主要以单体形式转运至质膜。这有力地支持了我们以前的发现,即TβRII可以作为单体存在于细胞膜上。我们还发现,配体刺激导致TβRII分子的传递速率提高和膜缔合时间延长。基于这些观察,我们提出了TGF-β1诱导的TβRII二聚化激活受体的机制。我们的方法为实时定量活细胞中细胞表面蛋白的空间排列,迁移率和寡聚化提供了有用的工具,











































 京公网安备 11010802027423号
京公网安备 11010802027423号