当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Elucidating the Structures of Amyloid Oligomers with Macrocyclic β-Hairpin Peptides: Insights into Alzheimer’s Disease and Other Amyloid Diseases
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-03-06 00:00:00 , DOI: 10.1021/acs.accounts.7b00554 Adam G. Kreutzer 1 , James S. Nowick 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-03-06 00:00:00 , DOI: 10.1021/acs.accounts.7b00554 Adam G. Kreutzer 1 , James S. Nowick 1
Affiliation
|
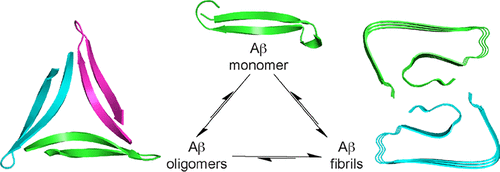
In the more than a century since its identification, Alzheimer’s disease has become the archetype of amyloid diseases. The first glimpses of the chemical basis of Alzheimer’s disease began with the identification of “amyloid” plaques in the brain in 1892 and extended to the identification of proteinaceous fibrils with “cross-β” structure in 1968. Further efforts led to the discovery of the β-amyloid peptide, Aβ, as a 40- or 42-amino acid peptide that is responsible for the plaques and fibrils. At this point, a three-decade-long marathon began to elucidate the structure of the fibrils and identify the molecular basis of Alzheimer’s disease. Along the way, an alternative model began to emerge in which small aggregates of Aβ, called “oligomers”, rather than fibrils, are the culprits that lead to neurodegeneration in Alzheimer’s disease. This Account describes what is known about the structures of the fibrils and details our research group’s efforts to understand the structural, biophysical, and biological properties of the oligomers in amyloid diseases.
中文翻译:

用大环β-发夹肽阐明淀粉样蛋白低聚物的结构:阿尔茨海默氏病和其他淀粉样蛋白疾病的见解。
自从被鉴定以来的一个多世纪中,阿尔茨海默氏病已成为淀粉样疾病的原型。阿尔茨海默氏病的化学基础的首次发现始于1892年在大脑中鉴定“淀粉样蛋白”斑块,并于1968年扩展到鉴定具有“cross-β”结构的蛋白原纤维。 β-淀粉样肽,Aβ,一种40或42个氨基酸的肽,负责斑块和原纤维。此时,长达三个十年的马拉松比赛开始阐明原纤维的结构并确定阿尔茨海默氏病的分子基础。在此过程中,一种替代模型开始出现,其中小的Aβ聚集体称为“寡聚体”,而不是原纤维,是导致阿尔茨海默氏病神经退行性变的元凶。
更新日期:2018-03-06
中文翻译:

用大环β-发夹肽阐明淀粉样蛋白低聚物的结构:阿尔茨海默氏病和其他淀粉样蛋白疾病的见解。
自从被鉴定以来的一个多世纪中,阿尔茨海默氏病已成为淀粉样疾病的原型。阿尔茨海默氏病的化学基础的首次发现始于1892年在大脑中鉴定“淀粉样蛋白”斑块,并于1968年扩展到鉴定具有“cross-β”结构的蛋白原纤维。 β-淀粉样肽,Aβ,一种40或42个氨基酸的肽,负责斑块和原纤维。此时,长达三个十年的马拉松比赛开始阐明原纤维的结构并确定阿尔茨海默氏病的分子基础。在此过程中,一种替代模型开始出现,其中小的Aβ聚集体称为“寡聚体”,而不是原纤维,是导致阿尔茨海默氏病神经退行性变的元凶。











































 京公网安备 11010802027423号
京公网安备 11010802027423号