当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Functional and Structural Analysis of Phenazine O-Methyltransferase LaPhzM from Lysobacter antibioticus OH13 and One-Pot Enzymatic Synthesis of the Antibiotic Myxin
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-03-06 00:00:00 , DOI: 10.1021/acschembio.8b00062 Jiasong Jiang 1 , Daisy Guiza Beltran , Andrew Schacht , Stephen Wright 1 , Limei Zhang , Liangcheng Du 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-03-06 00:00:00 , DOI: 10.1021/acschembio.8b00062 Jiasong Jiang 1 , Daisy Guiza Beltran , Andrew Schacht , Stephen Wright 1 , Limei Zhang , Liangcheng Du 1
Affiliation
|
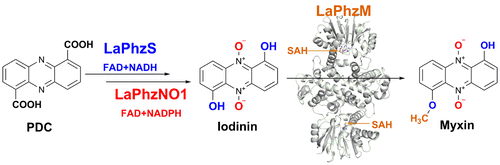
Myxin is a well-known antibiotic that had been used for decades. It belongs to the phenazine natural products that exhibit various biological activities, which are often dictated by the decorating groups on the heteroaromatic three-ring system. The three rings of myxin carry a number of decorations, including an unusual aromatic N5,N10-dioxide. We previously showed that phenazine 1,6-dicarboxylic acid (PDC) is the direct precursor of myxin, and two redox enzymes (LaPhzS and LaPhzNO1) catalyze the decarboxylative hydroxylation and aromatic N-oxidations of PDC to produce iodinin (1.6-dihydroxy-N5,N10-dioxide phenazine). In this work, we identified the LaPhzM gene from Lysobacter antibioticus OH13 and demonstrated that LaPhzM encodes a SAM-dependent O-methyltransferase converting iodinin to myxin. The results further showed that LaPhzM is responsible for both monomethoxy and dimethoxy formation in all phenazine compounds isolated from strain OH13. LaPhzM exhibits relaxed substrate selectivity, catalyzing O-methylation of phenazines with non-, mono-, or di-N-oxide. In addition, we demonstrated a one-pot biosynthesis of myxin by in vitro reconstitution of the three phenazine-ring decorating enzymes. Finally, we determined the X-ray crystal structure of LaPhzM with a bound cofactor at 1.4 Å resolution. The structure provided molecular insights into the activity and selectivity of the first characterized phenazine O-methyltransferase. These results will facilitate future exploitation of the thousands of phenazines as new antibiotics through metabolic engineering and chemoenzymatic syntheses.
中文翻译:

溶杆菌属细菌OH13的吩嗪O-甲基转移酶LaPhzM的功能和结构分析以及一锅酶法合成抗生素Myxin
Myxin是一种已使用了数十年的著名抗生素。它属于具有各种生物活性的吩嗪天然产物,通常由杂芳族三环系统上的修饰基团决定。粘菌素的三个环带有许多装饰,包括不寻常的芳香族N 5,N 10-二氧化物。我们以前表明,吩嗪1,6-二羧酸(PDC)是粘菌素的直接前体,并且两种氧化还原酶(LaPhzS和LaPhzNO1)催化PDC的脱羧羟基化和芳香族N-氧化,从而产生碘(1.6-dihydroxy- N 5,N 10-二氧化吩嗪)。在这项工作中,我们确定了LaPhzM从基因溶血杆菌抗生素OH13,并证明LaPhzM编码将iodinin转化为粘菌素的SAM依赖性O-甲基转移酶。结果进一步表明,LaPhzM负责从菌株OH13分离的所有吩嗪化合物中的单甲氧基和二甲氧基的形成。LaPhzM具有轻松的底物选择性,可通过非,一或二-N氧化物催化吩嗪的O-甲基化。此外,我们证明了通过体外一锅法生物合成粘菌素的方法三种吩嗪环修饰酶的重组。最后,我们确定了LaPhzM的X射线晶体结构,其结合的辅因子分辨率为1.4。该结构提供了对第一个表征的吩嗪O-甲基转移酶的活性和选择性的分子认识。这些结果将促进未来通过代谢工程和化学酶促合成方法开发成千上万的吩嗪类新抗生素。
更新日期:2018-03-06
中文翻译:

溶杆菌属细菌OH13的吩嗪O-甲基转移酶LaPhzM的功能和结构分析以及一锅酶法合成抗生素Myxin
Myxin是一种已使用了数十年的著名抗生素。它属于具有各种生物活性的吩嗪天然产物,通常由杂芳族三环系统上的修饰基团决定。粘菌素的三个环带有许多装饰,包括不寻常的芳香族N 5,N 10-二氧化物。我们以前表明,吩嗪1,6-二羧酸(PDC)是粘菌素的直接前体,并且两种氧化还原酶(LaPhzS和LaPhzNO1)催化PDC的脱羧羟基化和芳香族N-氧化,从而产生碘(1.6-dihydroxy- N 5,N 10-二氧化吩嗪)。在这项工作中,我们确定了LaPhzM从基因溶血杆菌抗生素OH13,并证明LaPhzM编码将iodinin转化为粘菌素的SAM依赖性O-甲基转移酶。结果进一步表明,LaPhzM负责从菌株OH13分离的所有吩嗪化合物中的单甲氧基和二甲氧基的形成。LaPhzM具有轻松的底物选择性,可通过非,一或二-N氧化物催化吩嗪的O-甲基化。此外,我们证明了通过体外一锅法生物合成粘菌素的方法三种吩嗪环修饰酶的重组。最后,我们确定了LaPhzM的X射线晶体结构,其结合的辅因子分辨率为1.4。该结构提供了对第一个表征的吩嗪O-甲基转移酶的活性和选择性的分子认识。这些结果将促进未来通过代谢工程和化学酶促合成方法开发成千上万的吩嗪类新抗生素。











































 京公网安备 11010802027423号
京公网安备 11010802027423号