Journal of Controlled Release ( IF 10.5 ) Pub Date : 2018-03-06 , DOI: 10.1016/j.jconrel.2018.03.004 Zhigao Niu , Eleni Samaridou , Emilie Jaumain , Julie Coëne , Gabriela Ullio , Neha Shrestha , Josep Garcia , Matilde Durán-Lobato , Sulay Tovar , Manuel J. Santander-Ortega , M. Victoria Lozano , M. Mar Arroyo-Jimenez , Rocío Ramos-Membrive , Iván Peñuelas , Aloïse Mabondzo , Véronique Préat , Meritxell Teixidó , Ernest Giralt , María José Alonso
|
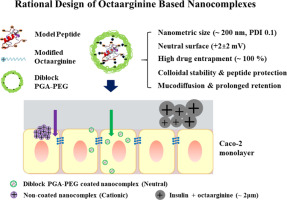
The objective of this work was the development of a new drug nanocarrier intended to overcome the barriers associated to the oral modality of administration and to assess its value for the systemic or local delivery of peptides. The nanocarrier was rationally designed taking into account the nature of the intestinal barriers and was loaded with insulin, which was selected as a model peptide. The nanocarrier consisted of a complex between insulin and a hydrophobically-modified cell penetrating peptide (CPP), enveloped by a protecting polymer. The selected CPP was octaarginine (r8), chemically conjugated with cholesterol (Chol) or lauric acid (C12), whereas the protecting polymer was poly (glutamic acid)-poly (ethylene glycol) (PGA-PEG). This enveloping material was intended to preserve the stability of the nanocomplex in the intestinal medium and facilitate its diffusion across the intestinal mucus. The enveloped nanocomplexes (ENCPs) exhibited a number of key features, namely (i) a unimodal size distribution with a mean size of 200 nm and a neutral zeta potential, (ii) the capacity to associate insulin (~100% association efficiency) and protect it from degradation in simulated intestinal fluids, (iii) the ability to diffuse through intestinal mucus and, most importantly, (iv) the capacity to interact with the Caco-2 model epithelium, resulting in a massive insulin cell uptake (47.59 ± 5.79%). This enhanced accumulation of insulin at the epithelial level was not translated into an enhanced insulin transport. In fact, only 2% of insulin was transported across the monolayer, and this was correlated with a moderate response of insulin following oral administration to healthy rats. Despite of this, the accumulation of the insulin-loaded nanocarriers in the intestinal mucosa could be verified in vivo upon their labeling with 99mTc. Overall, these data underline the capacity of the nanocarriers to overcome substantial barriers associated to the oral modality of administration and to facilitate the accumulation of the associated peptide at the intestinal level.
中文翻译:

PEG-PGA包裹的八精氨酸肽纳米复合物:口服肽递送策略
这项工作的目的是开发一种新的药物纳米载体,旨在克服与口服给药方式有关的障碍,并评估其对肽的全身或局部给药的价值。考虑到肠屏障的性质,对纳米载体进行了合理设计,并负载了胰岛素,胰岛素被选为模型肽。纳米载体由胰岛素和疏水修饰的细胞穿透肽(CPP)之间的复合物组成,并被保护性聚合物包裹。所选的CPP为八精氨酸(r8),与胆固醇(Chol)或月桂酸(C12)化学共轭,而保护聚合物为聚(谷氨酸)-聚(乙二醇)(PGA-PEG)。这种包封材料旨在保持纳米复合物在肠介质中的稳定性,并促进其在肠粘液中的扩散。包封的纳米复合物(ENCP)具有许多关键特征,即(i)具有200 nm平均大小和中性Zeta电位的单峰大小分布,(ii)缔合胰岛素的能力(〜100%缔合效率)和保护其免受模拟肠液降解的影响(iii)通过肠粘液扩散的能力,最重要的是(iv)与Caco-2模型上皮相互作用的能力,从而导致大量胰岛素细胞摄取(47.59±5.79) %)。胰岛素在上皮水平上的这种增强的积累未转化为胰岛素输送的增强。实际上,只有2%的胰岛素通过单层转运,这与健康大鼠口服胰岛素后的中度反应有关。尽管如此,可以证实在肠粘膜中载有胰岛素的纳米载体的积累在体内用99m Tc标记时。总的来说,这些数据强调了纳米载体克服与口服给药方式相关的实质性障碍并促进相关肽在肠内积累的能力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号