当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unimolecular decomposition of formamide via direct chemical dynamics simulations†
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-03-06 00:00:00 , DOI: 10.1039/c8cp00541a Anchal Gahlaut 1, 2, 3 , Manikandan Paranjothy 1, 2, 3
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-03-06 00:00:00 , DOI: 10.1039/c8cp00541a Anchal Gahlaut 1, 2, 3 , Manikandan Paranjothy 1, 2, 3
Affiliation
|
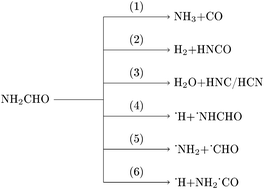
Formamide (NH2CHO), being the simplest organic molecule containing an amide functional group, serves as a prototype to study protein and peptide chemistry. Formamide has been found in Comets and interstellar media and its decomposition results in smaller molecules such as NH3, CO, HCN, HNCO, etc. These smaller molecules are considered to have been potential precursors for the formation of complex biological molecules, such as nucleic acids and nucleobases, in the early Earth. Several experimental and theoretical investigations of formamide decomposition have been reported in the literature. In the present work, unimolecular decomposition of formamide in the electronic ground state was investigated by classical direct chemical dynamics simulations. The calculations were performed at three different energies using the density functional B3LYP/aug-cc-pVDZ level of electronic structure theory. The major dissociation products observed were NH3, CO, H2, HNCO, H2O, HCN, and HNC along with products of a few minor dissociation channels. Reactivity, atomic level mechanisms, and product branching ratios were investigated as a function of total energy.
中文翻译:

通过直接化学动力学模拟 对甲酰胺进行单分子分解†
甲酰胺(NH 2 CHO)是含有酰胺官能团的最简单的有机分子,是研究蛋白质和肽化学的原型。在彗星和星际介质中发现了甲酰胺,其分解产生较小的分子,例如NH 3,CO,HCN,HNCO等。这些较小的分子被认为是在地球早期形成复杂的生物分子(例如核酸和核碱基)的潜在前体。文献中已经报道了一些甲酰胺分解的实验和理论研究。在目前的工作中,通过经典的直接化学动力学模拟研究了电子基态下甲酰胺的单分子分解。使用电子结构理论的密度泛函B3LYP / aug-cc-pVDZ能级,在三种不同的能量下进行了计算。观察到的主要离解产物为NH 3,CO,H 2,HNCO,H 2O,HCN和HNC以及一些次要解离通道的产物。研究了反应性,原子能级机理和产物支化比与总能量的关系。
更新日期:2018-03-06
中文翻译:

通过直接化学动力学模拟 对甲酰胺进行单分子分解†
甲酰胺(NH 2 CHO)是含有酰胺官能团的最简单的有机分子,是研究蛋白质和肽化学的原型。在彗星和星际介质中发现了甲酰胺,其分解产生较小的分子,例如NH 3,CO,HCN,HNCO等。这些较小的分子被认为是在地球早期形成复杂的生物分子(例如核酸和核碱基)的潜在前体。文献中已经报道了一些甲酰胺分解的实验和理论研究。在目前的工作中,通过经典的直接化学动力学模拟研究了电子基态下甲酰胺的单分子分解。使用电子结构理论的密度泛函B3LYP / aug-cc-pVDZ能级,在三种不同的能量下进行了计算。观察到的主要离解产物为NH 3,CO,H 2,HNCO,H 2O,HCN和HNC以及一些次要解离通道的产物。研究了反应性,原子能级机理和产物支化比与总能量的关系。











































 京公网安备 11010802027423号
京公网安备 11010802027423号