当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Predicting Nucleation of Isonicotinamide from the Solvent–Solute Interactions of Isonicotinamide in Common Organic Solvents
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-03-06 00:00:00 , DOI: 10.1021/acs.jpca.8b01342 Mark B. Lynch 1, 2 , Simon E. Lawrence 1 , Michael Nolan 2
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-03-06 00:00:00 , DOI: 10.1021/acs.jpca.8b01342 Mark B. Lynch 1, 2 , Simon E. Lawrence 1 , Michael Nolan 2
Affiliation
|
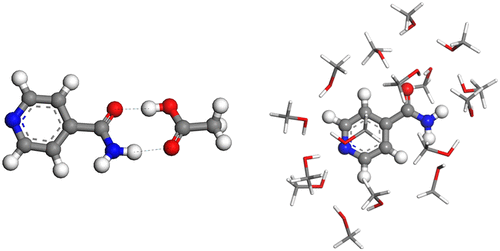
The interactions of isonicotinamide (INA) with seven common solvents (acetic acid, acetonitrile, acetone, chloroform, ethyl acetate, and methanol) have been studied to examine solute–solvent effects on the nucleation of INA from these solvents. In a simple model of 1:1 solute–solvent interactions, the strongest INA–solvent interaction is with acetic acid (binding energy, ΔEbind = −64.05 kJ mol–1) and the weakest is with chloroform (ΔEbind = −24.85 kJ mol–1). This arises since acetic acid and INA form a hydrogen-bonding motif containing two moderate strength N–H···O hydrogen bonds, while chloroform and INA have a single weak C–H···O hydrogen bond. Taking acetic acid, chloroform, and methanol, the solvents with the strongest, the weakest, and an intermediate strength INA–solvent binding energy, the solvation of INA was studied to compare it with the 1:1 model. Acetic acid has the strongest binding energy (−872.24 kJ mol–1) and solvation energy (−341.20 kJ mol–1) with chloroform binding energy (−517.72 kJ mol–1) and solvation energy (−199.05 kJ mol–1). Methanol has intermediate binding energy (−814.19 kJ mol–1) and solvation energies (−320.81 kJ mol–1). These results further confirm the recent the findings which indicate that the key trends in solvent–solute interactions can be determined from a simple and efficient 1:1 dimer model and can be used to predict ease of nucleation with stronger binding energies correlating to slower, more difficult nucleation. A limit of this model is revealed by considering alcohol and acid solvents with longer alkyl chains.
中文翻译:

从异烟酰胺在普通有机溶剂中的溶剂-相互作用相互作用预测异烟酰胺的成核
已研究了异烟酰胺(INA)与七种常见溶剂(乙酸,乙腈,丙酮,氯仿,乙酸乙酯和甲醇)的相互作用,以研究溶质-溶剂对这些溶剂对INA成核的影响。在1:1溶质-溶剂相互作用的简单模型中,最强的INA-溶剂相互作用是与乙酸(结合能,ΔE结合= -64.05 kJ mol –1),最弱的是与氯仿(ΔE结合=- 24.85 kJ mol –1)。这是由于乙酸和INA形成氢键基序,其中包含两个中等强度的N–H··O氢键,而氯仿和INA具有单个弱C–H···O氢键。以乙酸,氯仿和甲醇为溶剂,它们具有最强,最弱和中等强度的INA-溶剂结合能,研究了INA的溶剂化作用,并将其与1:1模型进行了比较。乙酸具有最强的结合能(−872.24 kJ mol –1)和溶剂化能(−341.20 kJ mol –1),具有氯仿结合能(−517.72 kJ mol –1)和溶剂化能(−199.05 kJ mol –1)。甲醇具有中间结合能(−814.19 kJ mol –1)和溶剂化能(−320.81 kJ mol–1)。这些结果进一步证实了最近的发现,这些发现表明溶剂-溶质相互作用的关键趋势可以通过简单而有效的1:1二聚体模型确定,并可以用来预测成核的容易性,结合能越强,则结合速度越慢,反应越快。难以成核。通过考虑具有更长烷基链的醇和酸溶剂,揭示了该模型的局限性。
更新日期:2018-03-06
中文翻译:

从异烟酰胺在普通有机溶剂中的溶剂-相互作用相互作用预测异烟酰胺的成核
已研究了异烟酰胺(INA)与七种常见溶剂(乙酸,乙腈,丙酮,氯仿,乙酸乙酯和甲醇)的相互作用,以研究溶质-溶剂对这些溶剂对INA成核的影响。在1:1溶质-溶剂相互作用的简单模型中,最强的INA-溶剂相互作用是与乙酸(结合能,ΔE结合= -64.05 kJ mol –1),最弱的是与氯仿(ΔE结合=- 24.85 kJ mol –1)。这是由于乙酸和INA形成氢键基序,其中包含两个中等强度的N–H··O氢键,而氯仿和INA具有单个弱C–H···O氢键。以乙酸,氯仿和甲醇为溶剂,它们具有最强,最弱和中等强度的INA-溶剂结合能,研究了INA的溶剂化作用,并将其与1:1模型进行了比较。乙酸具有最强的结合能(−872.24 kJ mol –1)和溶剂化能(−341.20 kJ mol –1),具有氯仿结合能(−517.72 kJ mol –1)和溶剂化能(−199.05 kJ mol –1)。甲醇具有中间结合能(−814.19 kJ mol –1)和溶剂化能(−320.81 kJ mol–1)。这些结果进一步证实了最近的发现,这些发现表明溶剂-溶质相互作用的关键趋势可以通过简单而有效的1:1二聚体模型确定,并可以用来预测成核的容易性,结合能越强,则结合速度越慢,反应越快。难以成核。通过考虑具有更长烷基链的醇和酸溶剂,揭示了该模型的局限性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号