当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fluorescent Carbon Quantum Dots with Intrinsic Nucleolus-Targeting Capability for Nucleolus Imaging and Enhanced Cytosolic and Nuclear Drug Delivery
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2018-03-06 00:00:00 , DOI: 10.1021/acsami.7b19549 Xian-Wu Hua , Yan-Wen Bao , Fu-Gen Wu
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2018-03-06 00:00:00 , DOI: 10.1021/acsami.7b19549 Xian-Wu Hua , Yan-Wen Bao , Fu-Gen Wu
|
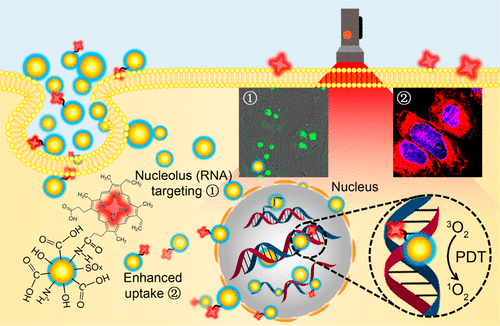
Nucleolus tracking and nucleus-targeted photodynamic therapy are attracting increasing attention due to the importance of nucleolus and the sensitivity of nucleus to various therapeutic stimuli. Herein, a new class of multifunctional fluorescent carbon quantum dots (or carbon dots, CDs) synthesized via the one-pot hydrothermal reaction of m-phenylenediamine and l-cysteine was reported to effectively target nucleolus. The as-prepared CDs possess superior properties, such as low-cost and facile synthesis, good water dispersibility, various surface groups for further modifications, prominent photostability, excellent compatibility, and rapid/convenient/wash-free staining procedures. Besides, as compared with SYTO RNASelect (a commonly used commercial dye for nucleolus imaging) that can only image nucleolus in fixed cells, the CDs can realize high-quality nucleolus imaging in not only fixed cells but also living cells, allowing the real-time tracking of nucleolus-related biological behaviors. Furthermore, after conjugating with protoporphyrin IX (PpIX), a commonly used photosensitizer, the resultant CD–PpIX nanomissiles showed remarkably increased cellular uptake and nucleus-targeting properties and achieved greatly enhanced phototherapeutic efficiency because the nuclei show poor tolerance to reactive oxygen species produced during the photodynamic therapy. The in vivo experiments revealed that the negatively charged CD–PpIX nanomissiles could rapidly and specifically target a tumor site after intravenous injection and cause efficient tumor ablation with no toxic side effects after laser irradiation. It is believed that the present CD-based nanosystem will hold great potential in nucleolus imaging and nucleus-targeted drug delivery and cancer therapy.
中文翻译:

具有内在核靶向能力的荧光碳量子点,用于核成像和增强的胞质和核药物传递
由于核仁的重要性以及核对各种治疗刺激的敏感性,核追踪和以核为靶标的光动力疗法引起了越来越多的关注。在本文中,一类新的通孔的一锅水热反应合成的多官能碳荧光量子点(或碳圆点,CDS)的米苯二胺和升据报道,β-半胱氨酸有效地靶向核仁。所制备的CD具有卓越的性能,例如低成本且易于合成,良好的水分散性,可进一步修饰的各种表面基团,出色的光稳定性,出色的相容性以及快速/便捷/免洗的染色程序。此外,与只能在固定细胞中对核仁成像的SYTO RNASelect(一种常用的用于核仁成像的商业染料)相比,CD不仅可以在固定细胞中而且还可以在活细胞中实现高质量的核仁成像,从而实现了实时成像。跟踪核仁相关的生物学行为。此外,在与常用的光敏剂原卟啉IX(PpIX)结合后,最终的CD–PpIX纳米导弹显示出显着增加的细胞摄取和细胞核靶向特性,并且由于细胞核对光动力疗法期间产生的活性氧种类的耐受性差,因此大大提高了光疗效率。体内实验表明,带负电荷的CD–PpIX纳米导弹在静脉内注射后可以快速,特异性地靶向肿瘤部位,并在激光照射后引起有效的肿瘤消融,而没有毒副作用。据信,目前的基于CD的纳米系统将在核仁成像以及以核为目标的药物递送和癌症治疗中具有巨大的潜力。体内实验表明,带负电荷的CD–PpIX纳米导弹在静脉内注射后可以快速,特异性地靶向肿瘤部位,并在激光照射后引起有效的肿瘤消融,而没有毒副作用。据信,目前的基于CD的纳米系统将在核仁成像以及以核为目标的药物递送和癌症治疗中具有巨大的潜力。体内实验表明,带负电荷的CD–PpIX纳米导弹在静脉内注射后可以快速,特异性地靶向肿瘤部位,并在激光照射后引起有效的肿瘤消融,而没有毒副作用。据信,目前的基于CD的纳米系统将在核仁成像以及以核为目标的药物递送和癌症治疗中具有巨大的潜力。
更新日期:2018-03-06
中文翻译:

具有内在核靶向能力的荧光碳量子点,用于核成像和增强的胞质和核药物传递
由于核仁的重要性以及核对各种治疗刺激的敏感性,核追踪和以核为靶标的光动力疗法引起了越来越多的关注。在本文中,一类新的通孔的一锅水热反应合成的多官能碳荧光量子点(或碳圆点,CDS)的米苯二胺和升据报道,β-半胱氨酸有效地靶向核仁。所制备的CD具有卓越的性能,例如低成本且易于合成,良好的水分散性,可进一步修饰的各种表面基团,出色的光稳定性,出色的相容性以及快速/便捷/免洗的染色程序。此外,与只能在固定细胞中对核仁成像的SYTO RNASelect(一种常用的用于核仁成像的商业染料)相比,CD不仅可以在固定细胞中而且还可以在活细胞中实现高质量的核仁成像,从而实现了实时成像。跟踪核仁相关的生物学行为。此外,在与常用的光敏剂原卟啉IX(PpIX)结合后,最终的CD–PpIX纳米导弹显示出显着增加的细胞摄取和细胞核靶向特性,并且由于细胞核对光动力疗法期间产生的活性氧种类的耐受性差,因此大大提高了光疗效率。体内实验表明,带负电荷的CD–PpIX纳米导弹在静脉内注射后可以快速,特异性地靶向肿瘤部位,并在激光照射后引起有效的肿瘤消融,而没有毒副作用。据信,目前的基于CD的纳米系统将在核仁成像以及以核为目标的药物递送和癌症治疗中具有巨大的潜力。体内实验表明,带负电荷的CD–PpIX纳米导弹在静脉内注射后可以快速,特异性地靶向肿瘤部位,并在激光照射后引起有效的肿瘤消融,而没有毒副作用。据信,目前的基于CD的纳米系统将在核仁成像以及以核为目标的药物递送和癌症治疗中具有巨大的潜力。体内实验表明,带负电荷的CD–PpIX纳米导弹在静脉内注射后可以快速,特异性地靶向肿瘤部位,并在激光照射后引起有效的肿瘤消融,而没有毒副作用。据信,目前的基于CD的纳米系统将在核仁成像以及以核为目标的药物递送和癌症治疗中具有巨大的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号