当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Total Synthesis of Lancifodilactone G Acetate. 2. Final Phase and Completion of the Total Synthesis
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-03-06 00:00:00 , DOI: 10.1021/acs.joc.7b02917 Kuang-Yu Wang 1 , Dong-Dong Liu 1 , Tian-Wen Sun 1 , Yong Lu 1 , Su-Lei Zhang 1 , Yuan-He Li 1 , Yi-Xin Han 1 , Hao-Yuan Liu 1 , Cheng Peng 1 , Qin-Yang Wang 1 , Jia-Hua Chen 1 , Zhen Yang 1, 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-03-06 00:00:00 , DOI: 10.1021/acs.joc.7b02917 Kuang-Yu Wang 1 , Dong-Dong Liu 1 , Tian-Wen Sun 1 , Yong Lu 1 , Su-Lei Zhang 1 , Yuan-He Li 1 , Yi-Xin Han 1 , Hao-Yuan Liu 1 , Cheng Peng 1 , Qin-Yang Wang 1 , Jia-Hua Chen 1 , Zhen Yang 1, 2
Affiliation
|
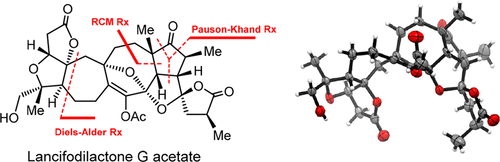
The asymmetric total synthesis of lancifodilactone G acetate was accomplished in 28 steps. The key steps in this synthesis include (i) an asymmetric Diels–Alder reaction for formation of the scaffold of the BC ring; (ii) an intramolecular ring-closing metathesis reaction for the formation of the trisubstituted cyclooctene using a Hoveyda–Grubbs II catalyst; (iii) an intramolecular Pauson–Khand reaction for construction of the sterically congested F ring; (iv) sequential cross-metathesis, hydrogenation, and lactonization reactions for installation of the anomerically stabilized bis-spiro ketal fragment of lancifodilactone G; and (v) a Dieckmann-type condensation reaction for installation of the A ring. The strategy and chemistry developed for the total synthesis will be useful in the synthesis of other natural products and complex molecules.
中文翻译:

Lancifodilactone G Acetate的不对称全合成。2.总合成的最终阶段和完成
lancifodilactone G Gacetate的不对称全合成过程需要28个步骤。该合成的关键步骤包括:(i)用于形成BC环骨架的不对称Diels-Alder反应;(ii)使用Hoveyda-Grubbs II催化剂进行分子内闭环复分解反应以形成三取代的环辛烯;(iii)分子内的Pauson-Khand反应,用于构建空间拥挤的F环;(iv)顺序的交叉复分解,氢化和内酯化反应,以安装Lancifodilactone G的阴离子稳定的双螺缩酮片段;(v)用于安装A环的狄克曼型缩合反应。为全合成而开发的策略和化学方法将对其他天然产物和复杂分子的合成有用。
更新日期:2018-03-06
中文翻译:

Lancifodilactone G Acetate的不对称全合成。2.总合成的最终阶段和完成
lancifodilactone G Gacetate的不对称全合成过程需要28个步骤。该合成的关键步骤包括:(i)用于形成BC环骨架的不对称Diels-Alder反应;(ii)使用Hoveyda-Grubbs II催化剂进行分子内闭环复分解反应以形成三取代的环辛烯;(iii)分子内的Pauson-Khand反应,用于构建空间拥挤的F环;(iv)顺序的交叉复分解,氢化和内酯化反应,以安装Lancifodilactone G的阴离子稳定的双螺缩酮片段;(v)用于安装A环的狄克曼型缩合反应。为全合成而开发的策略和化学方法将对其他天然产物和复杂分子的合成有用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号