当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Acid-Labile Degradation of Injectable Fiber Fragments to Release Bioreducible Micelles for Targeted Cancer Therapy
Biomacromolecules ( IF 5.5 ) Pub Date : 2018-03-02 00:00:00 , DOI: 10.1021/acs.biomac.7b01696 Zhoujiang Chen 1 , Weiping Liu 1 , Long Zhao 1 , Songzhi Xie 1 , Maohua Chen 1 , Tao Wang 1 , Xiaohong Li 1
Biomacromolecules ( IF 5.5 ) Pub Date : 2018-03-02 00:00:00 , DOI: 10.1021/acs.biomac.7b01696 Zhoujiang Chen 1 , Weiping Liu 1 , Long Zhao 1 , Songzhi Xie 1 , Maohua Chen 1 , Tao Wang 1 , Xiaohong Li 1
Affiliation
|
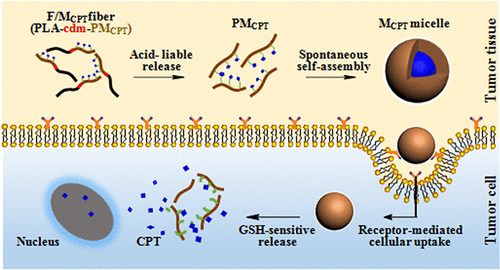
Cancer chemotherapy is confronted with difficulties enhancing the tumor accumulation, improving the bioavailability, and relieving the adverse effect of chemotherapeutic agents. To address the challenges, this study proposes a feasible strategy to realize a sustained release of drug-loaded micelles from fiber fragments after intratumoral injection. Camptothecin (CPT) is grafted on hyaluronic acid (HA) via 3,3′-dithiodipropionic acid to obtain reduction-sensitive promicelle polymers (PMCPT), which are conjugated with poly(d,l-lactide) via 2-propionic-3-methylmaleic anhydride (CDM) to obtain acid-labile copolymers for the preparation of injectable fiber fragments. Fiber fragments show remarkable acid-sensitive degradation, and the released PMCPT are spontaneously self-assembled into micelles, followed by subsequent HA-mediated internalization into tumor cells and reduction-sensitive release of drugs in the cytosol. Compared to fresh micelles prepared by ultrasonication, the micelles released via the degradation of fiber fragments display similar behaviors, such as the size and morphology, glutathione-sensitive drug release, cellular uptake efficiency, and cytotoxicity. Taking advantage of the aggregation-induced emission (AIE) effect of tetraphenylethene (TPE), the micelle release, cellular uptake, and tumor accumulation have been elucidated from the self-assembly induced fluorescence light-up in vitro and after intratumoral injection. Compared to the intratumoral injection of free micelles, sustained micelle release from fiber fragments resulted in significantly higher antitumor efficacy with respect to the inhibition of tumor growths, prolonging of animal survivals, and induction of cell apoptosis in tumor tissues. Thus, the micelle-releasing fiber fragments integrated with double targeting capabilities and double stimuli responsiveness have demonstrated a superior capacity to sustainably deliver chemotherapeutic agents directly within tumor cells.
中文翻译:

可注射纤维片段的酸不稳定降解,以释放可生物还原的胶束用于靶向癌症治疗
癌症化学疗法面临着增加肿瘤积累,提高生物利用度和减轻化学治疗剂的副作用的困难。为了解决这些挑战,这项研究提出了一种可行的策略,以实现在肿瘤内注射后从纤维碎片中持续释放载药胶束。喜树碱(CPT)通过3,3'-二硫代二丙酸接枝到透明质酸(HA)上,获得还原敏感性的胶束聚合物(PM CPT),该聚合物通过2-丙酸3与聚(d,1-丙交酯)共轭-甲基马来酸酐(CDM),以获得对酸不稳定的共聚物,用于制备可注射的纤维碎片。纤维碎片显示出显着的酸敏感性降解,并释放出PM CPT它们自发地自组装成胶束,随后通过HA介导的内在化进入肿瘤细胞,并在细胞溶质中降低敏感性释放药物。与通过超声处理制备的新鲜胶束相比,通过降解纤维碎片释放的胶束表现出相似的行为,例如大小和形态,谷胱甘肽敏感性药物释放,细胞吸收效率和细胞毒性。利用四苯乙烯(TPE)的聚集诱导发射(AIE)效应,已经从体外和肿瘤内注射后自组装诱导的荧光亮起阐明了胶束释放,细胞摄取和肿瘤蓄积。与瘤内注射游离胶束相比,从纤维碎片中持续释放胶束导致在抑制肿瘤生长,延长动物存活以及诱导肿瘤组织中细胞凋亡方面具有显着更高的抗肿瘤功效。因此,具有双重靶向能力和双重刺激响应性的整合的释放胶束的纤维片段显示出在直接在肿瘤细胞内可持续递送化学治疗剂的优异能力。
更新日期:2018-03-02
中文翻译:

可注射纤维片段的酸不稳定降解,以释放可生物还原的胶束用于靶向癌症治疗
癌症化学疗法面临着增加肿瘤积累,提高生物利用度和减轻化学治疗剂的副作用的困难。为了解决这些挑战,这项研究提出了一种可行的策略,以实现在肿瘤内注射后从纤维碎片中持续释放载药胶束。喜树碱(CPT)通过3,3'-二硫代二丙酸接枝到透明质酸(HA)上,获得还原敏感性的胶束聚合物(PM CPT),该聚合物通过2-丙酸3与聚(d,1-丙交酯)共轭-甲基马来酸酐(CDM),以获得对酸不稳定的共聚物,用于制备可注射的纤维碎片。纤维碎片显示出显着的酸敏感性降解,并释放出PM CPT它们自发地自组装成胶束,随后通过HA介导的内在化进入肿瘤细胞,并在细胞溶质中降低敏感性释放药物。与通过超声处理制备的新鲜胶束相比,通过降解纤维碎片释放的胶束表现出相似的行为,例如大小和形态,谷胱甘肽敏感性药物释放,细胞吸收效率和细胞毒性。利用四苯乙烯(TPE)的聚集诱导发射(AIE)效应,已经从体外和肿瘤内注射后自组装诱导的荧光亮起阐明了胶束释放,细胞摄取和肿瘤蓄积。与瘤内注射游离胶束相比,从纤维碎片中持续释放胶束导致在抑制肿瘤生长,延长动物存活以及诱导肿瘤组织中细胞凋亡方面具有显着更高的抗肿瘤功效。因此,具有双重靶向能力和双重刺激响应性的整合的释放胶束的纤维片段显示出在直接在肿瘤细胞内可持续递送化学治疗剂的优异能力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号