当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Protein-Derived Cofactors Revisited: Empowering Amino Acid Residues with New Functions
Biochemistry ( IF 2.9 ) Pub Date : 2018-03-02 00:00:00 , DOI: 10.1021/acs.biochem.8b00123 Victor L. Davidson 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-03-02 00:00:00 , DOI: 10.1021/acs.biochem.8b00123 Victor L. Davidson 1
Affiliation
|
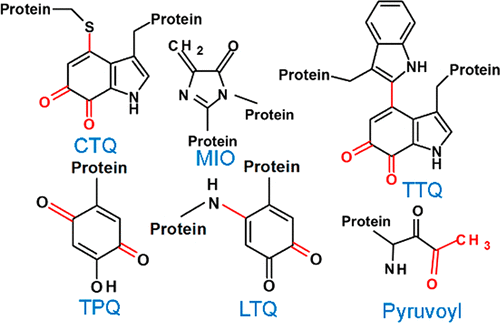
A protein-derived cofactor is a catalytic or redox-active site in a protein that is formed by post-translational modification of one or more amino acid residues. These post-translational modifications are irreversible and endow the modified amino acid residues with new functional properties. This Perspective focuses on the following advances in this area that have occurred during recent years. The biosynthesis of the tryptophan tryptophylquinone cofactor is catalyzed by a diheme enzyme, MauG. A bis-FeIV redox state of the hemes performs three two-electron oxidations of specific Trp residues via long-range electron transfer. In contrast, a flavoenzyme catalyzes the biosynthesis of the cysteine tryptophylquinone (CTQ) cofactor present in a newly discovered family of CTQ-dependent oxidases. Another carbonyl cofactor, the pyruvoyl cofactor found in classes of decarboxylases and reductases, is formed during an apparently autocatalytic cleavage of a precursor protein at the N-terminus of the cleavage product. It has been shown that in at least some cases, the cleavage is facilitated by binding to an accessory protein. Tyrosylquinonine cofactors, topaquinone and lysine tyrosylquinone, are found in copper-containing amine oxidases and lysyl oxidases, respectively. The physiological roles of different families of these enzymes in humans have been more clearly defined and shown to have significant implications with respect to human health. There has also been continued characterization of the roles of covalently cross-linked amino acid side chains that influence the reactivity of redox-active metal centers in proteins. These include Cys-Tyr species in galactose oxidase and cysteine dioxygenase and the Met-Tyr-Trp species in the catalase-peroxidase KatG.
中文翻译:

再谈蛋白质衍生的辅助因子:赋予氨基酸残基新功能
蛋白质衍生的辅因子是蛋白质中的催化或氧化还原活性位点,是通过翻译后修饰一个或多个氨基酸残基形成的。这些翻译后修饰是不可逆的,并使修饰的氨基酸残基具有新的功能特性。本观点着眼于近年来在该领域取得的以下进展。色氨酸色氨酸提花醌辅助因子的生物合成由二血红素酶MauG催化。双铁IV血红素的氧化还原态通过远程电子转移对特定的Trp残基进行三个两电子氧化。相反,黄素酶催化新发现的依赖于CTQ的氧化酶家族中的半胱氨酸色氨酸醌(CTQ)辅助因子的生物合成。另一种羰基辅因子,即在脱羧酶和还原酶类中发现的丙酮酰辅因子,是在裂解产物的N端明显地自催化裂解前体蛋白的过程中形成的。已经表明,至少在某些情况下,通过与辅助蛋白结合而促进了切割。在含铜胺氧化酶和赖氨酰氧化酶中分别发现了酪氨酰奎宁辅助因子,托帕醌和赖氨酸酪氨酰醌。这些酶的不同家族在人类中的生理作用已得到更清晰的定义,并显示出对人类健康的重要影响。还持续表征了影响蛋白质中氧化还原活性金属中心反应性的共价交联氨基酸侧链的作用。这些包括半乳糖氧化酶和半胱氨酸双加氧酶中的Cys-Tyr物种和过氧化氢酶-过氧化物酶KatG中的Met-Tyr-Trp物种。
更新日期:2018-03-02
中文翻译:

再谈蛋白质衍生的辅助因子:赋予氨基酸残基新功能
蛋白质衍生的辅因子是蛋白质中的催化或氧化还原活性位点,是通过翻译后修饰一个或多个氨基酸残基形成的。这些翻译后修饰是不可逆的,并使修饰的氨基酸残基具有新的功能特性。本观点着眼于近年来在该领域取得的以下进展。色氨酸色氨酸提花醌辅助因子的生物合成由二血红素酶MauG催化。双铁IV血红素的氧化还原态通过远程电子转移对特定的Trp残基进行三个两电子氧化。相反,黄素酶催化新发现的依赖于CTQ的氧化酶家族中的半胱氨酸色氨酸醌(CTQ)辅助因子的生物合成。另一种羰基辅因子,即在脱羧酶和还原酶类中发现的丙酮酰辅因子,是在裂解产物的N端明显地自催化裂解前体蛋白的过程中形成的。已经表明,至少在某些情况下,通过与辅助蛋白结合而促进了切割。在含铜胺氧化酶和赖氨酰氧化酶中分别发现了酪氨酰奎宁辅助因子,托帕醌和赖氨酸酪氨酰醌。这些酶的不同家族在人类中的生理作用已得到更清晰的定义,并显示出对人类健康的重要影响。还持续表征了影响蛋白质中氧化还原活性金属中心反应性的共价交联氨基酸侧链的作用。这些包括半乳糖氧化酶和半胱氨酸双加氧酶中的Cys-Tyr物种和过氧化氢酶-过氧化物酶KatG中的Met-Tyr-Trp物种。











































 京公网安备 11010802027423号
京公网安备 11010802027423号