当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An “On-Cycle” Precatalyst Enables Room-Temperature Polyfluoroarylation Using Sensitive Boronic Acids
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-03-02 00:00:00 , DOI: 10.1021/acscatal.8b00341 Liye Chen 1 , Haydn Francis 1 , Brad P. Carrow 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-03-02 00:00:00 , DOI: 10.1021/acscatal.8b00341 Liye Chen 1 , Haydn Francis 1 , Brad P. Carrow 1
Affiliation
|
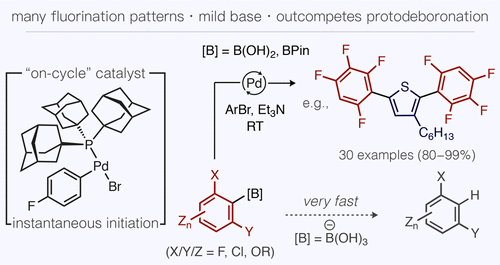
The use of fluorinated arylboronic acid building blocks in cross-coupling has remained challenging, because of their acute base sensitivity. We report a general solution to this problem using a true catalytic intermediate, Pd(PAd3)(p-FC6H4)Br, as a uniquely effective “on-cycle” precatalyst that allows Suzuki–Miyaura coupling to occur much faster than even the most severe protodeboronation side reactions. Control of boron speciation between the active acid and dormant ester forms was also found to play a critical role in balancing the rates of catalysis versus reagent decomposition. This method is compatible with any fluorination pattern, base-labile functional groups, and a range of bromo(hetero)arenes.
中文翻译:

“循环”预催化剂可使用敏感的硼酸实现室温下的多氟芳基化
交叉偶联中使用氟化芳基硼酸结构单元仍然具有挑战性,因为它们具有敏锐的碱敏感性。我们报告了使用真正的催化中间体Pd(PAd 3)(p -FC 6 H 4)Br作为唯一有效的“循环”预催化剂,可以使Suzuki-Miyaura偶联发生的速度快得多的一般解决方案。即使是最严重的原去硼副反应。还发现控制活性酸形式和休眠酯形式之间的硼形态在平衡催化速率与试剂分解之间起着至关重要的作用。该方法与任何氟化模式,对碱不稳定的官能团以及一系列溴(杂)芳烃均兼容。
更新日期:2018-03-02
中文翻译:

“循环”预催化剂可使用敏感的硼酸实现室温下的多氟芳基化
交叉偶联中使用氟化芳基硼酸结构单元仍然具有挑战性,因为它们具有敏锐的碱敏感性。我们报告了使用真正的催化中间体Pd(PAd 3)(p -FC 6 H 4)Br作为唯一有效的“循环”预催化剂,可以使Suzuki-Miyaura偶联发生的速度快得多的一般解决方案。即使是最严重的原去硼副反应。还发现控制活性酸形式和休眠酯形式之间的硼形态在平衡催化速率与试剂分解之间起着至关重要的作用。该方法与任何氟化模式,对碱不稳定的官能团以及一系列溴(杂)芳烃均兼容。











































 京公网安备 11010802027423号
京公网安备 11010802027423号