当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Self-Assembling Benzothiazole-Based Gelators: A Mechanistic Understanding of in Vitro Bioactivation and Gelation
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-03-05 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b01106 Francesca Citossi 1 , Thomas Smith 1 , Jong Bong Lee 1 , Joel Segal 1 , Pavel Gershkovich 1 , Michael J. Stocks 1 , Tracey D. Bradshaw 1 , Barrie Kellam 1 , Maria Marlow 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-03-05 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b01106 Francesca Citossi 1 , Thomas Smith 1 , Jong Bong Lee 1 , Joel Segal 1 , Pavel Gershkovich 1 , Michael J. Stocks 1 , Tracey D. Bradshaw 1 , Barrie Kellam 1 , Maria Marlow 1
Affiliation
|
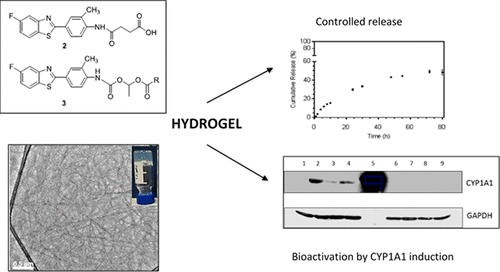
Low molecular weight gelators (LMWGs) of chemotherapeutic drugs represent a valid alternative to the existing polymer-based formulations used for targeted delivery of anticancer drugs. Herein we report the design and development of novel self-assembling gelators of the antitumor benzothiazole 5F 203 (1). Two different types of derivatives of 1 were synthesized, formed by an amide (2) and a carbamate (3a–3d) linker, respectively, which showed potent in vitro antitumor activity against MCF-7 mammary and IGROV-1 ovarian carcinoma cells. In contrast, MRC-5 fibroblasts were inherently resistant to the above derivatives (GI50 > 10 μM), thus revealing stark selectivity against the malignant cell lines over the nontransformed fibroblasts. Western blots assays demonstrated induction of CYP1A1 by 1 and its derivatives only in sensitive malignant cells (MCF-7), corroborating conservation of a CYP1A1-mediated mechanism of action. The ability to form stable gels under relatively high strains was supported by rheological tests; in addition, their inner morphology was characterized as possessing a crossed-linked nanostructure, with the formation of thick aggregates with variable widths between 1100 and 400 nm and lengths from 8 to 32 μm. Finally, in vitro dissolution studies proved the ability of hydrogel 2 to release 48% of 2 within 80 h, therefore demonstrating its ability to act as a platform for localized delivery.
中文翻译:

自组装基于苯并噻唑的胶凝剂:体外生物激活和胶凝的机械理解。
化学疗法药物的低分子量胶凝剂(LMWG)代表了用于靶向递送抗癌药物的现有基于聚合物的配方的有效替代品。在本文中,我们报告了抗肿瘤苯并噻唑5F 203(1)新型自组装胶凝剂的设计和开发。合成了两种不同类型的1衍生物,分别由酰胺(2)和氨基甲酸酯(3a - 3d)接头形成,它们显示出对MCF-7乳腺和IGROV-1卵巢癌细胞有效的体外抗肿瘤活性。相反,MRC-5成纤维细胞固有地对上述衍生物具有抗性(GI 50> 10μM),从而显示出相对于未转化的成纤维细胞对恶性细胞系的鲜明选择性。Western blots检测证明CYP1A1仅在敏感的恶性细胞(MCF-7)中被1及其衍生物诱导,从而证实了CYP1A1介导的作用机制的保守性。流变测试证实了在较高应变下形成稳定凝胶的能力。另外,它们的内部形态被表征为具有交联的纳米结构,形成厚的聚集体,其宽度在1100至400nm之间,长度在8至32μm之间。最后,体外溶出研究证明水凝胶2能够释放48%的2 80小时之内,因此证明了它可以充当本地交付平台。
更新日期:2018-03-05
中文翻译:

自组装基于苯并噻唑的胶凝剂:体外生物激活和胶凝的机械理解。
化学疗法药物的低分子量胶凝剂(LMWG)代表了用于靶向递送抗癌药物的现有基于聚合物的配方的有效替代品。在本文中,我们报告了抗肿瘤苯并噻唑5F 203(1)新型自组装胶凝剂的设计和开发。合成了两种不同类型的1衍生物,分别由酰胺(2)和氨基甲酸酯(3a - 3d)接头形成,它们显示出对MCF-7乳腺和IGROV-1卵巢癌细胞有效的体外抗肿瘤活性。相反,MRC-5成纤维细胞固有地对上述衍生物具有抗性(GI 50> 10μM),从而显示出相对于未转化的成纤维细胞对恶性细胞系的鲜明选择性。Western blots检测证明CYP1A1仅在敏感的恶性细胞(MCF-7)中被1及其衍生物诱导,从而证实了CYP1A1介导的作用机制的保守性。流变测试证实了在较高应变下形成稳定凝胶的能力。另外,它们的内部形态被表征为具有交联的纳米结构,形成厚的聚集体,其宽度在1100至400nm之间,长度在8至32μm之间。最后,体外溶出研究证明水凝胶2能够释放48%的2 80小时之内,因此证明了它可以充当本地交付平台。











































 京公网安备 11010802027423号
京公网安备 11010802027423号