当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Evaluation of Pazopanib Phase Behavior Following pH-Induced Supersaturation
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-03-05 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00081 Hikaru Sugihara 1, 2 , Lynne S. Taylor 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-03-05 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00081 Hikaru Sugihara 1, 2 , Lynne S. Taylor 1
Affiliation
|
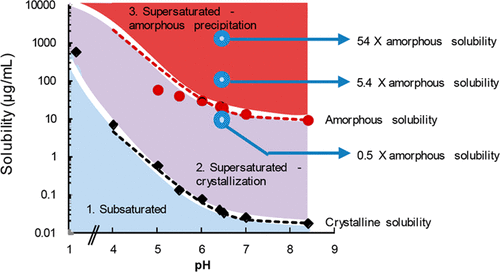
Salts of weakly basic active pharmaceutical ingredients are widely used to improve aqueous solubility and/or dissolution rate. However, these compounds are prone to precipitation due to the lower solubility of the un-ionized species at the higher pH in the intestinal region, and this can result in poor and/or variable absorption. The goal of this study was to investigate the degree of supersaturation achieved following dissolution of different amounts of pazopanib hydrochloride at low pH, followed by rapid pH increase. Using pH solubility profiles, phase boundaries were defined for crystalline and amorphous free base forms. The resultant phase diagram was used to rationalize the observed supersaturation and phase behavior of pazopanib following pH adjustment. In the presence of a crystallization inhibitor, hydroxypropylmethyl cellulose (HPMC), the degree of supersaturation was found to be very high, approximately 600-fold, at pH 6.5. At a dose equivalent to the clinical dose, the maximum free drug concentration observed at pH 6.5 was dictated by the amorphous solubility. Solutions that exceeded the amorphous solubility upon pH increase were found to undergo glass–liquid phase separations (GLPS) with the formation of amorphous colloidal drug-rich particles. Microscopic observations confirmed that HPMC delayed the appearance of pazopanib free base crystals. The phase behavior upon pH change is thus well predicted by the phase diagram, after taking into consideration the initial dose, the extent of supersaturation generated upon pH change, and the presence or absence of a crystallization inhibitor.
中文翻译:

pH诱导的过饱和后帕唑帕尼相行为的评估
弱碱性活性药物成分的盐被广泛用于改善水溶性和/或溶解速率。但是,由于这些化合物在肠道较高的pH值下在肠道中的溶解度较低,因此易于沉淀,这可能导致吸收效果差和/或变化。这项研究的目的是研究在低pH值下溶解不同量的盐酸帕唑帕尼后,pH值快速增加后所达到的过饱和度。使用pH溶解度曲线,定义了晶态和无定形游离碱形式的相界。所得相图用于合理调节pH后观察到的帕唑帕尼的过饱和度和相行为。在结晶抑制剂羟丙基甲基纤维素(HPMC)的存在下,发现在pH 6.5时过饱和度非常高,约为600倍。在等于临床剂量的剂量下,pH 6.5时观察到的最大游离药物浓度由无定形溶解度决定。发现在pH升高时超过无定形溶解度的溶液会经历玻璃-液相分离(GLPS),并形成无定形胶体药物富集颗粒。显微镜观察证实,HPMC延迟了帕唑帕尼游离碱晶体的出现。因此,在考虑初始剂量,pH改变时产生的过饱和程度以及是否存在结晶抑制剂后,通过相图可以很好地预测pH改变时的相行为。在等于临床剂量的剂量下,pH 6.5时观察到的最大游离药物浓度由无定形溶解度决定。发现在pH升高时超过无定形溶解度的溶液会经历玻璃-液相分离(GLPS),并形成无定形胶体药物富集颗粒。显微镜观察证实,HPMC延迟了帕唑帕尼游离碱晶体的出现。因此,在考虑初始剂量,pH改变时产生的过饱和程度以及是否存在结晶抑制剂后,通过相图可以很好地预测pH改变时的相行为。在等于临床剂量的剂量下,pH 6.5时观察到的最大游离药物浓度由无定形溶解度决定。发现在pH升高时超过无定形溶解度的溶液会经历玻璃-液相分离(GLPS),并形成无定形胶体药物富集颗粒。显微镜观察证实,HPMC延迟了帕唑帕尼游离碱晶体的出现。因此,在考虑初始剂量,pH改变时产生的过饱和程度以及是否存在结晶抑制剂后,通过相图可以很好地预测pH改变时的相行为。发现在pH升高时超过无定形溶解度的溶液会经历玻璃-液相分离(GLPS),并形成无定形胶体药物富集颗粒。显微镜观察证实,HPMC延迟了帕唑帕尼游离碱晶体的出现。因此,在考虑初始剂量,pH改变时产生的过饱和程度以及是否存在结晶抑制剂后,通过相图可以很好地预测pH改变时的相行为。发现在pH升高时超过无定形溶解度的溶液会经历玻璃-液相分离(GLPS),并形成无定形胶体药物富集颗粒。显微镜观察证实,HPMC延迟了帕唑帕尼游离碱晶体的出现。因此,在考虑初始剂量,pH改变时产生的过饱和程度以及是否存在结晶抑制剂后,通过相图可以很好地预测pH改变时的相行为。
更新日期:2018-03-05
中文翻译:

pH诱导的过饱和后帕唑帕尼相行为的评估
弱碱性活性药物成分的盐被广泛用于改善水溶性和/或溶解速率。但是,由于这些化合物在肠道较高的pH值下在肠道中的溶解度较低,因此易于沉淀,这可能导致吸收效果差和/或变化。这项研究的目的是研究在低pH值下溶解不同量的盐酸帕唑帕尼后,pH值快速增加后所达到的过饱和度。使用pH溶解度曲线,定义了晶态和无定形游离碱形式的相界。所得相图用于合理调节pH后观察到的帕唑帕尼的过饱和度和相行为。在结晶抑制剂羟丙基甲基纤维素(HPMC)的存在下,发现在pH 6.5时过饱和度非常高,约为600倍。在等于临床剂量的剂量下,pH 6.5时观察到的最大游离药物浓度由无定形溶解度决定。发现在pH升高时超过无定形溶解度的溶液会经历玻璃-液相分离(GLPS),并形成无定形胶体药物富集颗粒。显微镜观察证实,HPMC延迟了帕唑帕尼游离碱晶体的出现。因此,在考虑初始剂量,pH改变时产生的过饱和程度以及是否存在结晶抑制剂后,通过相图可以很好地预测pH改变时的相行为。在等于临床剂量的剂量下,pH 6.5时观察到的最大游离药物浓度由无定形溶解度决定。发现在pH升高时超过无定形溶解度的溶液会经历玻璃-液相分离(GLPS),并形成无定形胶体药物富集颗粒。显微镜观察证实,HPMC延迟了帕唑帕尼游离碱晶体的出现。因此,在考虑初始剂量,pH改变时产生的过饱和程度以及是否存在结晶抑制剂后,通过相图可以很好地预测pH改变时的相行为。在等于临床剂量的剂量下,pH 6.5时观察到的最大游离药物浓度由无定形溶解度决定。发现在pH升高时超过无定形溶解度的溶液会经历玻璃-液相分离(GLPS),并形成无定形胶体药物富集颗粒。显微镜观察证实,HPMC延迟了帕唑帕尼游离碱晶体的出现。因此,在考虑初始剂量,pH改变时产生的过饱和程度以及是否存在结晶抑制剂后,通过相图可以很好地预测pH改变时的相行为。发现在pH升高时超过无定形溶解度的溶液会经历玻璃-液相分离(GLPS),并形成无定形胶体药物富集颗粒。显微镜观察证实,HPMC延迟了帕唑帕尼游离碱晶体的出现。因此,在考虑初始剂量,pH改变时产生的过饱和程度以及是否存在结晶抑制剂后,通过相图可以很好地预测pH改变时的相行为。发现在pH升高时超过无定形溶解度的溶液会经历玻璃-液相分离(GLPS),并形成无定形胶体药物富集颗粒。显微镜观察证实,HPMC延迟了帕唑帕尼游离碱晶体的出现。因此,在考虑初始剂量,pH改变时产生的过饱和程度以及是否存在结晶抑制剂后,通过相图可以很好地预测pH改变时的相行为。











































 京公网安备 11010802027423号
京公网安备 11010802027423号